
Home / News
-
-
-
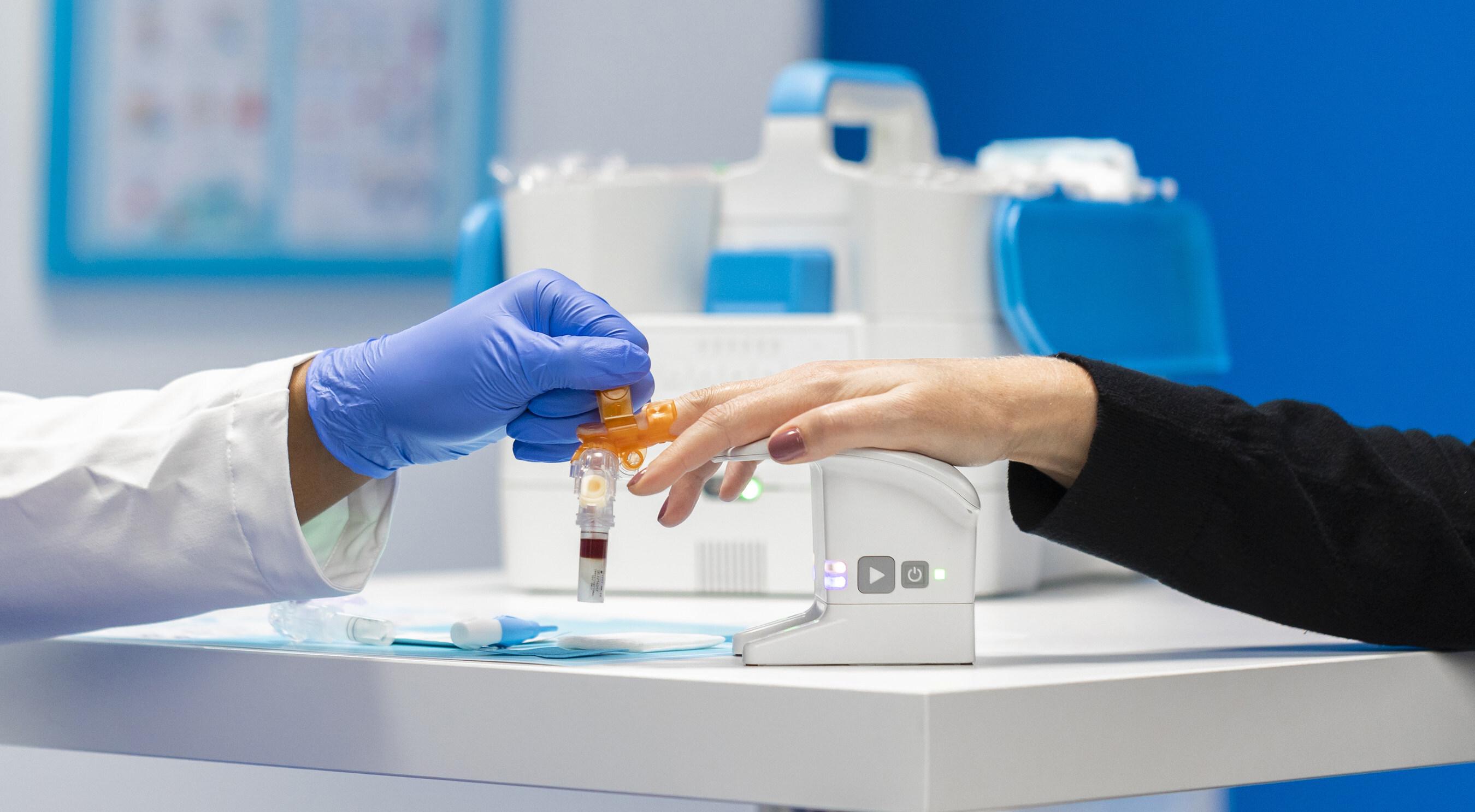
BD (Becton, Dickinson and Company) (NYSE: BDX), a leading global medical technology company, and Babson Diagnostics, a science-first health care technology company, today announced an expansion of fingertip blood collection and testing technologies for use by U.S. health systems and other large provider networks in settings like urgent cares, doctor offices and other ambulatory care settings.2024-12-18View More
-
-
-

-
North China's Tianjin Municipality issued a license for medical services on Monday to a wholly foreign-owned, third-grade general hospital, the first of its kind in the country.2024-12-17View More
-
-
-

-
2.3 Pathogen Diagnosis of Gastroenteritis Gastroenteritis is a common disease in infants and young children. The treatment of mild cases is based on the prevention and correction of dehydration, electrolyte dist..2024-12-17View More
-
-
-

-
Cardio Diagnostics Holdings, Inc. (NASDAQ: CDIO), an AI-driven precision cardiovascular medicine company today announced that the Company¡¯s PrecisionCHD and Epi+Gen CHD tests have received final pricing determinations from the Centers for Medicare & Medicaid Services (CMS). Following the preliminary pricing determination made by CMS in August 2024, CMS finalized the ¡®gapfill¡¯ pricing determination for both PrecisionCHD and Epi+Gen CHD. This decision will be effective for claims with dates of service on or after January 1, 2025, and will allow Medicare contractors to determine pricing for PrecisionCHD and Epi+Gen CHD based on actual cost data from Cardio Diagnostics. The Medicare contractors will report to CMS preliminary gapfill pricing for calendar year 2025 by April 1, 2025.2024-12-17View More
-
-
-

-
QIAGEN (NYSE: QGEN, Frankfurt Prime Standard: QIA) today announced the launch of Ingenuity Pathway Analysis (IPA) Interpret, a new feature designed to simplify and accelerate the interpretation of complex biological data.2024-12-16View More
-
-
-

-
Roche (SIX: RO, ROG; OTCQX: RHHBY) announced today the CE certification of the new cobas® 6800/8800 systems 2.0. The update significantly enhances the efficiency of laboratories by optimising resources, reducing downtime, consolidating test menus, and increasing throughput. These improvements ultimately promise a more streamlined diagnostics experience for healthcare professionals and their patients.2024-12-16View More
-
-
-

-
The World Health Organization (WHO) has achieved a milestone by approving the addition of a packaging and shipping site for a WHO prequalified HIV rapid test for professional use. This landmark approval enables the test to be procured by governments across Africa and major international organizations, such as the Global Fund and the President¡¯s Emergency Plan for AIDS Relief (PEPFAR).2024-12-13View More
-
-
-

-
Labcorp, a global leader of innovative and comprehensive laboratory services, announces it has finalized its acquisition of select outreach laboratory services from Ballad Health. The sale of these lab services, combined with Ballad Health¡¯s ongoing strategic relationship with Labcorp, aims to enhance patient care, expand access to advanced testing and improve efficiency for the health system and its patients.2024-12-13View More
-
-
-

-
South Korean diagnostic firm Noul said Wednesday that it has inked an agreement to launch its hematology and malaria testing tools in Europe through German lab chain Limbach Group.2024-12-12View More
-
-
FDA Grants Breakthrough Device Designation to MeMed Severity Test for Patients with Suspected Sepsis
-

-
MeMed, a leader in the emerging field of advanced host response technologies, today announced that the FDA has granted Breakthrough Device Designation (BDD) to its innovative MeMed Severity™ test. This designation underscores the transformative potential of MeMed Severity to advance the management of patients with suspected acute infections and suspected sepsis by empowering clinicians with timely, data-driven, clinical insights.2024-12-12View More
-
-
-

-
On 5 December, Lei Haichao, Director General of China's National Health Commission (NHC), met with Saudi Health Minister Fahad Abdulrahman AlJalajel in Beijing for an in-depth discussion on enhancing bilateral health ..2024-12-11View More
-
-
-

-
Akoya Biosciences on Tuesday said it has inked an exclusive global license agreement with German molecular diagnostics firm NeraCare to develop and commercialize NeraCare¡¯s Immunoprint test for early-stage melanoma on Akoya's immunofluorescence platform.2024-12-11View More
-
-
-

-
Agilent Technologies Inc. (NYSE: A) today announced the issuing of a Class C companion diagnostic In Vitro Diagnostic Regulation (IVDR) certification for PD-L1 IHC 28-8 pharmDx (Code SK005). This CDx assay has previously been CE-IVD¨Cmarked for sales in the European Union and is now certified in accordance with the new EU Regulation for in vitro diagnostic medical devices (IVDR) 1. PD-L1 IHC 28-8 pharmDx is approved for exclusive use with the Agilent Autostainer Link 48 advanced staining solution.2024-12-11View More
-
-
-

-
Natera, Inc. (NASDAQ: NTRA), a global leader in cell-free DNA and genetic testing, and MyOme, a leading clinical whole genome analysis and polygenic risk modeling company, today announced the launch of an integrated polygenic risk score (iPRS) for personalized breast cancer risk assessment. iPRS, which reports 5-year and lifetime breast cancer risk, offers individuals who receive a negative test result with Natera¡¯s Empower hereditary cancer test the opportunity for further risk assessment using MyOme¡¯s integrated polygenic risk score.2024-12-10View More
-
-
-

-
bioM¨¦rieux, a world leader in the field of in vitro diagnostics, today announces that its BIOFIRE® FILMARRAY® Tropical Fever (TF) Panel has received U.S. Food and Drug Administration (FDA) Special 510(k) clearance. This innovative polymerase-chain reaction (PCR) testing solution offers fast and accurate pathogen identification in patients with unexplained fever, helping to optimize treatment overall.2024-12-10View More
-
-
-

-
2. Molecular Diagnostic Reagents for Infectious Diseases 2.1 Latent Tuberculosis Infection Detection Reagents Latent tuberculosis infection (LTBI) is defined as a state of persistent immune respons..2024-12-09View More
-
-
-

-
Veracyte, Inc. (Nasdaq: VCYT), a leading cancer diagnostics company, today announced that its market-leading Decipher Prostate Genomic Classifier is the only gene expression test to be included in version 1 of the 2025 NCCN* Clinical Practice Guidelines in Oncology (NCCN Guidelines®) as part of the updated ¡°Advanced Tools¡± table located in the Principles of Risk Stratification and Biomarkers section (PROS-H) .2024-12-09View More
-
-
-

-
Copan Diagnostics, a leader in microbiology laboratory innovations, is proud to announce that UriSponge®, a urine collection and transport device which uses a new formulation of advanced preservatives to ensure specimen stability for culture, has received clearance from the U.S. Food and Drug Administration (FDA). The solution offers clinical laboratories and healthcare providers a user-friendly, reliable, and cost-effective way to improve urine specimen collection, preservation, and transport.2024-12-09View More
-
-
-

-
The company says the new facility will be the ¡°modern diagnostics production center in Europe.¡±2024-12-06View More
-
-
-

-
The World Health Organization (WHO) has granted prequalification to the molecular diagnostic test for tuberculosis (TB) called Xpert® MTB/RIF Ultra. It is the first test for TB diagnosis and antibiotic susceptibility testing that meets WHO's prequalification standards.2024-12-06View More
-

- CAIVD WeChat
Subscription Account

- CAIVD WeChat
Channels
China Association of In-vitro Diagnostics
Part of the information in our website is from the internet.
If by any chance it violates your rights, please contact us.

