
Home / News
-
-

-
BD (Becton, Dickinson and Company) (NYSE: BDX) ("BD" or the "Company") today announced that the Company's Board of Directors has set the close of business on February 5, 2026, as the record date for the previously announced spin-off of BD's Biosciences & Diagnostic Solutions business to BD's shareholders. Immediately following the spin-off, the spun-off entity will be combined with Waters Corporation (NYSE: WAT) ("Waters") in a Reverse Morris Trust transaction. The combination is expected to be completed on February 9, 2026, subject to the satisfaction of customary closing conditions.2026-01-28View More
-
-
-

-
The instruments employed by hospitals participating in the basic study were mainly from various manufacturers such as Sysmex, Stago, Werfen, Sekisui, Beckman, Mindray, Succeeder, BSBE, and Rayto, involving 96 instrume..2026-01-27View More
-
-
-
-
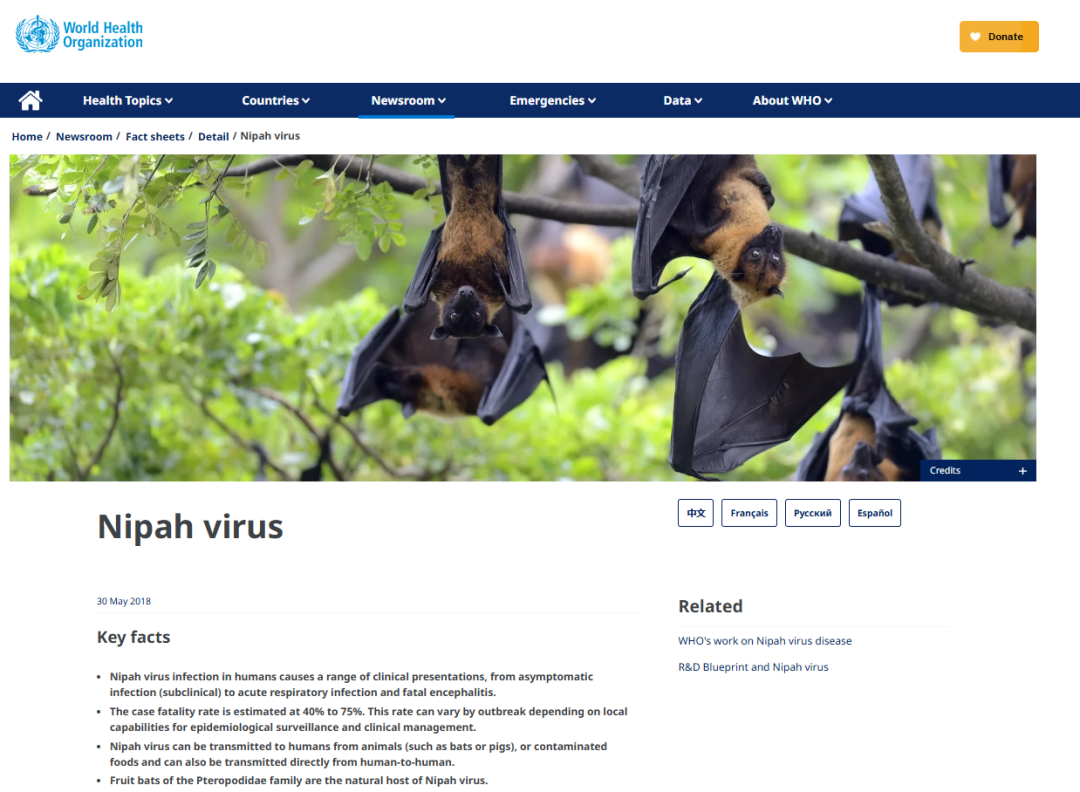
Recently, an outbreak of Nipah virus occurred in West Bengal, India, with 5 confirmed cases reported, including several healthcare workers. Nearly 100 people have been placed under home quarantine, and one patient is ..2026-01-27View More
-
-
-

-
The Fujian Agriculture and Forestry University in East China has unveiled the country's first humanoid diagnostic and treatment robot that deeply integrates non-invasive brain computer interface (BCI) technology. The robot is expected to help address long-standing challenges in early intervention of autism and other neurodevelopmental disorders, bringing good hope to the rehabilitation of more than 13 million autism patients in China, Global Times learned from the robot developers on Sunday.2026-01-27View More
-
-
-

-
Diasorin (FTSE MIB: DIA) announced today that, following the recent 510(k) clearance and CLIA-waiver from the U.S. Food and Drug Administration (FDA) for its first assay¡ªthe FLU A/B, RSV & COVID-19 Panel for use on the LIAISON NES® platform¡ª the Company has entered into an exclusive distribution agreement with Fisher Scientific, part of Thermo Fisher Scientific, for the LIAISON NES® molecular point-of-care (POC) platform in the U.S. hospital market.2026-01-27View More
-
-
-

-
Qure.ai, a global digital health company focused on AI-led medical diagnostics, has received a multi-million-dollar grant from the Gates Foundation to support the development of AI-enabled point-of-care ultrasound and create an open-source multi-modal health database aimed at improving lung disease detection in underserved regions.2026-01-26View More
-
-
-

-
Merck (MRK.N), opens new tab is no longer in discussions to buy cancer drug developer Revolution Medicines (RVMD.O), opens new tab, the Wall Street Journal reported on Sunday.2026-01-26View More
-
-
-

-
Guardant Health, Inc. (Nasdaq: GH), a leading precision oncology company, today announced that the U.S. Food and Drug Administration (FDA) has approved Guardant360® CDx as a companion diagnostic to identify patients with BRAF V600E-mutant metastatic colorectal cancer (mCRC) who may benefit from treatment with BRAFTOVI® (encorafenib) in combination with cetuximab and chemotherapy in accordance with the approved product labeling.2026-01-23View More
-
-
-

-
HORIBA, a global leader in analytical and measurement technology, has obtained CE IVDR certification for its new Yumizen H500 CRP benchtop hematology analyzer which delivers a simultaneous 5-part extended differential (DIFF) and rapid C-Reactive Protein (CRP) whole blood measurement. Based on its award winning Yumizen H500 compact analyzer, the new instrument is designed for small laboratories and ideal for use in a variety of hospital testing settings for rapid and highly accurate detection of infection and inflammation.2026-01-23View More
-
-
-

-
BIOMAKERS, a precision medicine and oncology intelligence company headquartered in San Francisco, today announced the closing of an additional $8 million. The financing marks a key inflection point as the company transitions from building a differentiated global diagnostics and data infrastructure to scaling an AI-native platform ¨C while continuing to expand its comprehensive molecular testing reach across Latin America ¨C designed to accelerate drug and diagnostic development, improve clinical trial execution, and enable precision oncology at global scale.2026-01-22View More
-
-
-

-
Truvian Health (¡°Truvian¡±), a diagnostics company redefining routine blood testing, today announced U.S. Food and Drug Administration (FDA) clearance for the Complete Blood Count (CBC) on its TruVerus™ multi‑modal blood testing system (K251249).2026-01-22View More
-
-
-

-
In November 2025, China's National Health Commission and four other authorities have called for the broader application of artificial intelligence (AI) in the country's health sector over the coming years. ..2026-01-21View More
-
-
-

-
Roche's (ROG.S), opens new tab Genentech said on Tuesday it will more than double its initial investment in a biomanufacturing facility in North Carolina to about $2 billion, as the companies look to further boost their $50 billion investment in the U.S.2026-01-21View More
-
-
-

-
Guardant Health, Inc. (Nasdaq: GH), a leading precision oncology company, today announced a multi-year collaboration with Merck, known as MSD outside the United States and Canada, to support the development and commercialization of Merck¡¯s oncology portfolio using the Guardant Infinity™ Smart platform.2026-01-20View More
-
-
-

-
2.1.1 Brand Research and Analysis on the Use of Blood Coagulation Analyzers in Different Medical Institutions According to the results of the basic research (Fig. 2), different medical institutions have ..2026-01-20View More
-
-
-

-
BD (Becton, Dickinson and Company) (NYSE: BDX), a leading global medical technology company, today announced that the U.S. Food and Drug Administration (FDA) has granted 510(k) clearance for the EnCor EnCompass™ Breast Biopsy and Tissue Removal System, a state-of-the-art multi-modality breast biopsy system designed to provide clinicians with flexibility across breast imaging modalities in the diagnosis of breast disease.2026-01-19View More
-
-
-

-
Autobio Diagnostics¡¯ hs-cTnT Assay Recognized by IFCC as Global Performance Benchmark Autobio Diagnostics recently announced that its high-sensitivity cardiac troponin T (hs-cTnT) assay (magnetic partic..2026-01-19View More
-
-
-

-
BillionToOne, Inc. (NASDAQ: BLLN), a next-generation molecular diagnostics company with a mission to create powerful and accurate tests that are accessible to all, today announced a collaboration with Epic, the nation's most widely used comprehensive electronic health record (EHR). The agreement, signed in December 2025, will integrate BillionToOne's prenatal and oncology testing portfolio with Epic's Aura diagnostics suite.2026-01-19View More
-
-
-

-
BGI Genomics (300676.SZ), opens new tab and Roche Diagnostics (ROG.S), opens new tab have rolled out tests for Alzheimer's disease in China, the companies said, in an effort to expand access to easier-to-use diagnosis and monitoring choices for patients with the brain-wasting condition.2026-01-16View More
-
-
-

-
Roche Diagnostics¡¯ MTB Nucleic Acid Test Kit and Domestic cobas 5800 Molecular Diagnostic System Receive NMPA Approval Roche Diagnostics China announced that the cobas® MTB Mycobacterium tuberculosi..2026-01-16View More
-

- CAIVD WeChat
Subscription Account

- CAIVD WeChat
Channels
China Association of In-vitro Diagnostics
Part of the information in our website is from the internet.
If by any chance it violates your rights, please contact us.

