
Chinese IVD Companies Assist in Nipah Virus Detection
2026/1/27 18:27:49 Views£º283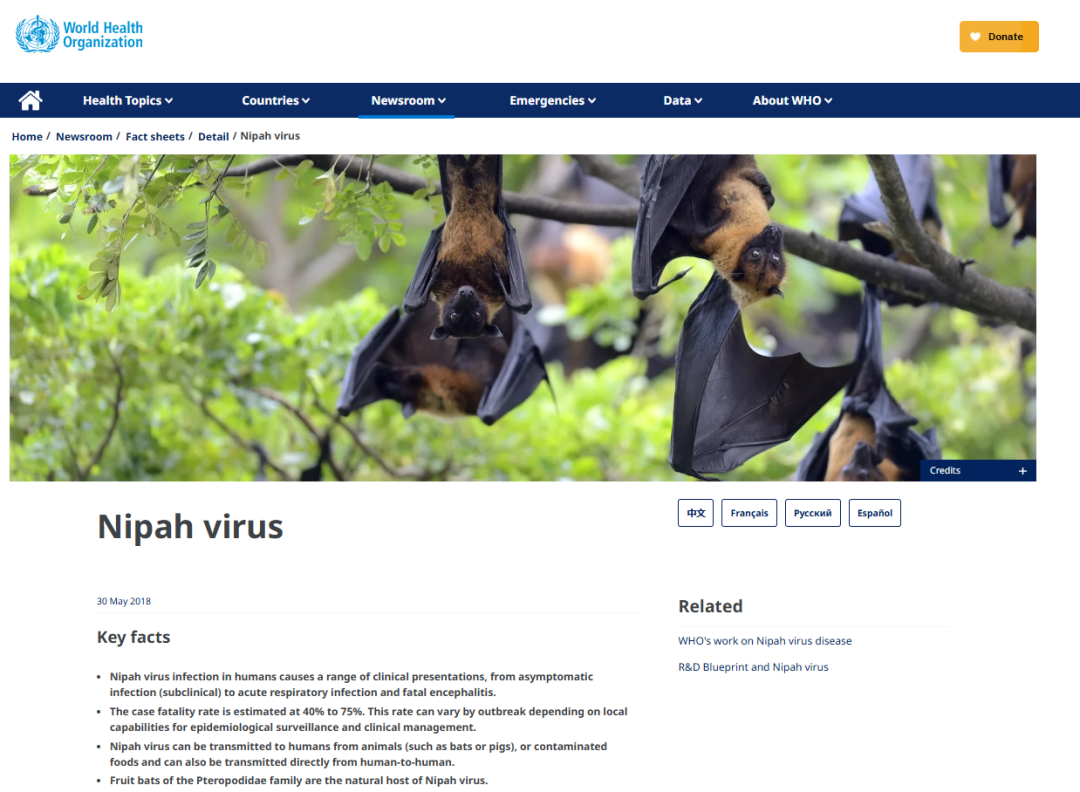
Recently, an outbreak of Nipah virus occurred in West Bengal, India, with 5 confirmed cases reported, including several healthcare workers. Nearly 100 people have been placed under home quarantine, and one patient is in critical condition, highlighting the urgency of epidemic control. As a lethal zoonotic virus with a fatality rate of 40%¨C75%, Nipah virus has no specific vaccine or antiviral treatment. Its transmission routes are complex, capable of spreading from animals to humans through contact with host animal fluids (such as fruit bats) or consumption of contaminated raw date palm sap or fruits, as well as from human to human via close contact with respiratory secretions or saliva. Early monitoring and rapid screening are crucial to curbing the spread of the outbreak.
Autobio

Autobio's fully automated nucleic acid purification and real-time fluorescent PCR analysis system, AutoMolec 3000/1600, employs classic magnetic bead-based nucleic acid extraction and fluorescent quantitative PCR technology, providing "sample-in, result-out" rapid detection support. The company has developed a viral nucleic acid rapid detection kit based on the fluorescent PCR method, enabling accurate screening for single or multiple targets.
Bioperfectus

With over a decade of expertise in disease prevention and control, Bioperfectus has actively participated in the containment of major epidemics. In response to the sudden outbreak of Nipah virus in India, Bioperfectus promptly supplied Nipah virus nucleic acid detection kits, comprehensively supporting epidemic monitoring and prevention efforts by customs and disease control authorities.
Hybribio
Hybribio, with years of dedication to genetic testing, remains focused on emergency responses to public health crises. Addressing the recent outbreak in India, Hybribio independently developed a Nipah virus nucleic acid detection kit (fluorescent PCR method), offering precise detection performance to support global port quarantine and disease control surveillance, thereby strengthening the first line of defense against the epidemic.
Liferiver
Liferiver is one of the early domestic companies to obtain CE certification for a Nipah virus nucleic acid detection kit (RT-PCR method, approved in 2022). The kit has been included in the procurement list of Indian government laboratories and is directly used for case confirmation.
Zhongke Shengyi

Zhongke Shengyi's Nipah virus nucleic acid detection kit enables on-site rapid testing and differential diagnosis. Utilizing a microfluidic integrated chip reaction system and real-time fluorescent PCR technology with target-specific primers and TaqMan probes, the kit performs fully automated rapid detection via a handheld real-time fluorescent quantitative PCR integrated system. The entire process, from sample processing to result analysis, completes within 56 minutes, with a sensitivity of 100 copies/reaction.
Vipotion
In the face of the severe Nipah virus outbreak, rapid screening and accurate diagnosis are key to containing its spread. Leveraging robust R&D capabilities and technical expertise, Vipotion has introduced a Nipah virus (NIPAH) nucleic acid detection kit (fluorescent PCR method) in response to the sudden epidemic in India, contributing valuable time to epidemic control efforts.
Mabsky
Confronted with the critical situation of the Nipah virus outbreak, Mabsky, drawing on years of technical experience, offers a high-sensitivity and high-specificity Nipah virus (with internal control) nucleic acid detection solution. This provides comprehensive and efficient testing support for medical institutions, disease control centers, and research units.

- CAIVD WeChat
Subscription Account

- CAIVD WeChat
Channels
China Association of In-vitro Diagnostics
Part of the information in our website is from the internet.
If by any chance it violates your rights, please contact us.

