
Home / News
-
-

-
On July 6, 2021, Agilent Technologies (NYSE: A) announced a strategic collaboration with GenomiCare Biotechnology in a move aimed to advance the application of the latest Next Generation Sequencing (NGS) technology into the clinical and scientific arena. More specifically, the purpose of using advanced NGS technology was to accelerate the process of development of genetic testing and related products and services, and to bring high level of clinical diagnosis and treatment of cancer in China.2021-07-09View More
-
-
-

-
Quidel has issued a recall for certain lots of its Lyra brand SARS-CoV-2 tests due to the potential for false negative results in patient samples with relatively high amounts of virus.2021-07-08View More
-
-
-

-
Global life sciences leader Cytiva has acquired Intermountain Life Sciences, a manufacturer of high-purity water, buffers, and liquid cell culture media. Cytiva will use Intermountain¡¯s manufacturing site in Utah to rapidly boost its liquid cell culture production by millions of liters.2021-07-07View More
-
-
-

-
On June 30, Sichuan Orienter Biotechnology Co., Ltd. and InTec PRODUCTS INC. applied for the Growth Enterprise Market and was accepted by Shenzhen Stock Exchange.2021-07-06View More
-
-
-

-
The US Food and Drug Administration recently granted separate Emergency Use Authorization for molecular SARS-CoV-2 tests from Exact Sciences and BioGx, an immunoassay for detecting the virus from Bio-Rad Laboratories, and an at-home specimen collection kit from Everlywell.2021-07-05View More
-
-
-

-
NEW YORK ¨C Qiagen said on Thursday that it has formed a global strategic alliance with Japan's Sysmex to develop and commercialize cancer companion diagnostics using Sysmex's Plasma-Safe-SeqS next-generation sequencing technology.2021-07-02View More
-
-
-
-
On the evening of June 30, it is said that Hotgen Biotech will increase by about £¤1.378 billion yuan to about £¤1.598 billion yuan, with a year-on-year increase of 70546.05% to 81808.47% ,which is Compared with the same period of last year.2021-07-02View More
-
-
-

-
Recently, South Korean idol Song Joong Ki endorsed SD BIOSENSOR, an in vitro diagnostic company, sparking heated discussions in the IVD industry.2021-07-01View More
-
-
-
-
To date, no immunological data on COVID-19 heterologous vaccination schedules in humans have been reported. We assessed the immunogenicity and reactogenicity of BNT162b2 (Comirnaty, BioNTech, Mainz, Germany) administered as second dose in participants primed with ChAdOx1-S (Vaxzevria, AstraZeneca, Oxford, UK).2021-06-30View More
-
-
-

-
On June 21st, COVID-19 Antigen Rapid Test (Colloidal Gold) COVID-19 Antigen Schnelltest (kolloidales Gold) Probe: Speichel (Spucktest) developed by Joinstar Biomedical Technology obtained the approval of the German Federal Agency for Medicines and Medical Devices to enter the self-testing list.2021-06-29View More
-
-
-

-
On June 26, 2021, Shanghai, Sanyou Biopharmaceutical (Shanghai) Co., Ltd. announced the closing of series B financing. This financing round was co-led by LH Ventures and Montesy Investment and followed by Grand Mount Capital and Seeking Capital (an existing investor).2021-06-28View More
-
-
-

-
WALTHAM, Mass.¨C(BUSINESS WIRE)¨CJun. 22, 2021¨C PerkinElmer, Inc. (NYSE: PKI) (¡°PerkinElmer¡±) is pleased to announce it has entered into an agreement to acquire SIRION Biotech GmbH, a leading, global provider of viral vector-based technologies that drive improved delivery performance for cell and gene therapies. The acquisition is expected to close during the third quarter of 20212021-06-25View More
-
-
-
-
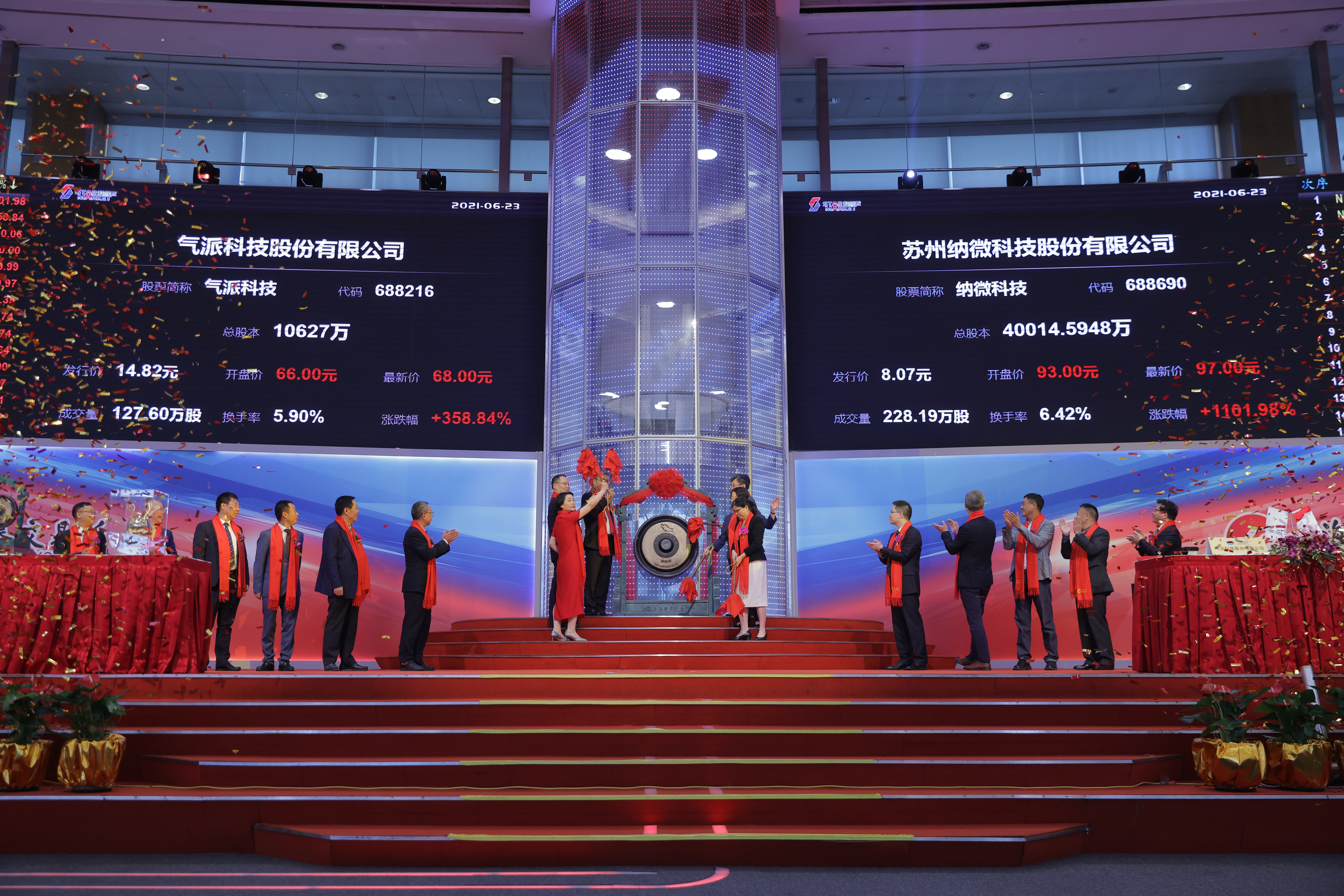
On June 23, 2021, Suzhou Nanomicro Technology Co., Ltd. was listed on SSE STAR Market. Stock code: 688690. The number of A shares publicly issued this time is 44 million shares, the issue price is 8.07 yuan/share, and the opening price is 93.00 yuan/share.2021-06-24View More
-
-
-

-
On the evening of June 21st, Sansure Biotech acquired a 14.77% equity interest in GeneMind Biosciences through equity transfer and subscription of newly added registered capital at a cost of RMB 255.2 million. Sansure Biotech will complete the transaction with its own funds. When the transaction is completed, Sansure Biotech will become the second largest shareholder of GeneMind Biosciences.2021-06-23View More
-
-
-

-
JP Morgan and Cowen said on Monday they have initiated coverage of shares of Singular Genomics Systems.JP Morgan gave the shares an Overweight rating and a price target of $30 while Cowen gave them an Outperform rating.2021-06-22View More
-
-
-

-
Roche announced recently that its RT-PCR-based Cobas SARS-CoV-2 nucleic acid test for use on the Cobas Liat system has received Emergency Use Authorization from the US Food and Drug Administration.2021-06-21View More
-
-
-

-
A new antibody testing study examining samples originally collected through the National Institutes of Health¡¯s All of Us Research Program found evidence of SARS-CoV-2 infections in five states earlier than had initially been reported.2021-06-21View More
-
-
-

-
WASHINGTON, June 17, 2021 /PRNewswire/ -- Danaher Corporation (NYSE: DHR) (the "Company") announced today that Danaher has entered into a definitive agreement to acquire privately held Aldevron, for a cash purchase price of approximately $9.6 billion. Danaher expects to finance the acquisition using cash on hand and/or proceeds from the issuance of commercial paper.2021-06-18View More
-
-
-

-
Exponent announces that it has reached an agreement to sell BBI Group (¡°BBI¡±), a leading supplier of products and services to the global diagnostics and life sciences industries, to Novo Holdings A/S, a leading global life sciences investor, for an enterprise value of over ¡ê400 million.2021-06-17View More
-
-
-
-
On June 16, 2021, AVE Science & Technology Co. Ltd. was listed on the Sci-Tech innovation board. Stock abbreviation is AVE Science & Technology. Stock code is 688067. The number of A shares publicly issued this time is 17 million shares, the issue price is 14.71 yuan/share, the opening price is 38.24 yuan/share, and the maximum increase at the time of press is 207%.2021-06-16View More
-

- CAIVD WeChat
Subscription Account

- CAIVD WeChat
Channels
China Association of In-vitro Diagnostics
Part of the information in our website is from the internet.
If by any chance it violates your rights, please contact us.

