
Home / News
-
-

-
Sansure biology and Zhuhai Baolian signed the termination agreement of the share transfer agreement on August 5, 2021.2021-08-06View More
-
-
-

-
2021 ranking features 143 Chinese companies, 122 U.S. companies, and 53 Japanese companies. 6 in vitro diagnostic companies on the list.2021-08-05View More
-
-
-

-
On August 3, Sinopharm listed its 30% equity interest in Maixin Biotechnology on the China Beijing Equity Exchange at a price of 947.5 million yuan. Beijing Strong Biotechnologies, Inc. (hereinafter referred to as BSBE) said they would fight for the acquisition.2021-08-04View More
-
-
-

-
DiaSorin said on Friday that its second quarter revenues rose 20 percent year over year, driven by strength in molecular diagnostic test sales.2021-08-03View More
-
-
-

-
On July 28, 2021, Thermo Fisher Scientific announcing that it would invest in the construction of another brand-new factory under Thermo Fisher China manufacturing center in Suzhou High Tech Zone. The total investment is $50 million, which is expected to be completed and put into use in 2022.2021-07-30View More
-
-
-

-
PerkinElmer, Inc. (NYSE: PKI) (¡°PerkinElmer¡±) today announced it has entered into an agreement to acquire BioLegend, a leading, global provider of life science antibodies and reagents, for approximately $5.25 billion in a combination of cash and stock, subject to certain adjustments.2021-07-28View More
-
-
-

-
PerkinElmer, Inc. (NYSE: PKI), a global leader committed to innovating for a healthier world, today reported financial results for the second quarter ended July 4, 2021.2021-07-28View More
-
-
-

-
On July 25, 2021, Autobio and Syscan officially announced the launch of a strategic cooperation. In the future, the two companies will make full use of their respective technology and channel advantages to carry out a full range of in-depth cooperation in the field of coagulation testing.2021-07-27View More
-
-
-

-
July 22, Roche reported a 51 percent increase in half-year revenues for its Diagnostics division, largely thanks to demand for its COVID-19 products.2021-07-27View More
-
-
-

-
Danaher reported before the opening of the market on Thursday that its second quarter revenues rose 37 percent year over year, thanks largely to steep gains from its life sciences and diagnostics businesses.2021-07-27View More
-
-
-

-
July 22, Abbott said that its Diagnostics business revenues grew 63 percent year over year in the second quarter, while its total revenues were up 39 percent.2021-07-27View More
-
-
-
-
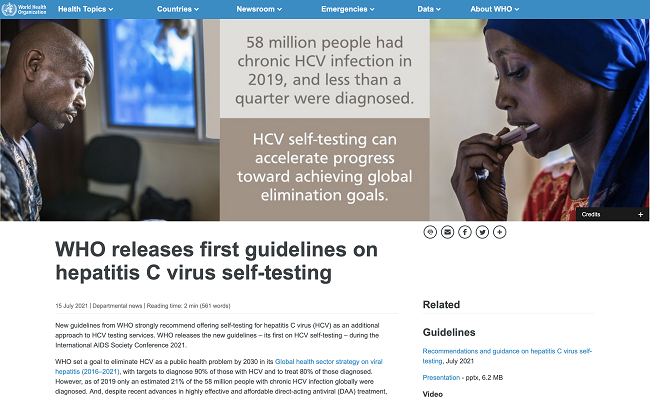
New guidelines from WHO strongly recommend offering self-testing for hepatitis C virus (HCV) as an additional approach to HCV testing services. WHO releases the new guidelines ¨C its first on HCV self-testing ¨C during the International AIDS Society Conference 2021.2021-07-23View More
-
-
-
-
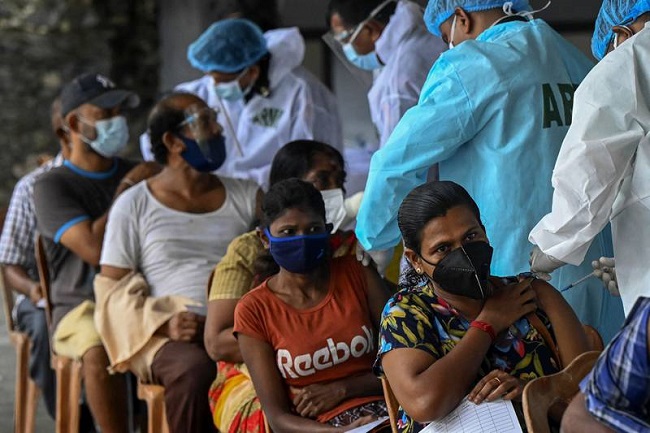
Research in Sri Lanka shows more than 80 per cent of those inoculated develop antibodies capable of neutralising the virus2021-07-22View More
-
-
-
-
Agilent Technologies on Tuesday announced that it has received CE marking for the expanded use of its PD-L1 IHC 22C3 pharmDx companion diagnostic assay in patients with non-small cell lung cancer.2021-07-21View More
-
-
-

-
Runda Medical issued a performance forecast on the evening of July 19, and it is expected that the net profit attributable to shareholders of listed companies will be between 195 million yuan and 215 million yuan in the first half of 2021, an increase of 83.78 million yuan to 104 million yuan compared with the same period of the previous year, an increase of 75.33% year-on-year to 93.31%.2021-07-20View More
-
-
-
-
Recently, Fosun Pharma announced that the approval of the mRNA novel coronal vaccine by the Food and Drug Administration has been basically completed, and the expert review has passed. The administrative approval stage is being stepped up at present.2021-07-19View More
-
-
-
-
On July 14, China Securities Regulatory Commission (CSRC) released the news and approved the IPO registration of Sino Biological, Inc. Sino Biological is an international reagent supplier and service provider, which specializes in recombinant protein production and antibody development.2021-07-16View More
-
-
-

-
Medix Biochemica, a global leader in critical IVD raw materials, has acquired all of the shares of Diaclone SAS ("Diaclone"). Through acquisition, Medix Biochemica broadens its portfolio of critical raw materials for the in-vitro diagnostics ("IVD") industry and expands its global network.2021-07-15View More
-
-
-

-
On July 9th, Roche Diagnostics Asia-Pacific production base and R&D center built another new manufacturing building-System Reagent Manufacturing Base. It was officially completed in Suzhou Industrial Park. The total investment in the project is 390 million yuan. In the future, the system reagent products manufactured by it will be applied to the modular instruments and pathology laboratory instruments of Roche Diagnostics' central laboratories in China and other countries in the Asia-Pacific region.2021-07-13View More
-
-
-
-
Recently, it was learned from Shanghai Fosun Pharma that Fosun Industrial (Hong Kong) Co., Ltd., a subsidiary of Fosun Pharma Holding, officially signed a sales agreement with Taiwan Semiconductor Manufacturing Company, FOXCONN, YongLin, and Zuellig Pharma, respectively, to sell a total of 10 million doses of COVID-mRNA vaccine to Zuellig Pharma, which was commissioned by TSMC, FOXCONN, and YongLin. This batch of vaccine will be donated by the buyers to disease control agencies in Taiwan for local vaccination.2021-07-12View More
-

- CAIVD WeChat
Subscription Account

- CAIVD WeChat
Channels
China Association of In-vitro Diagnostics
Part of the information in our website is from the internet.
If by any chance it violates your rights, please contact us.

