
Home / News
-
-

-
Original from: PR Newswire ¡¤ The test, run on Abbott's portable i-STAT® Alinity® instrument, uses whole blood to help evaluate patients with a suspected mild traumatic brain injury (mTBI), other..2025-06-17View More
-
-
-

-
bioM¨¦rieux, a world leader in the field of in vitro diagnostics, announces an agreement to acquire the assets of Day Zero Diagnostics, a US-based infectious disease diagnostics company using genome sequencing and machine learning to combat the rise of antibiotic-resistant infections. This strategic acquisition aims to enhance bioM¨¦rieux's capabilities in next-generation sequencing (NGS) and rapid diagnostics, further solidifying its commitment to advancing healthcare and Antimicrobial Stewardship through innovative solutions.2025-06-17View More
-
-
-

-
University of Chicago spinout OrisDx said Tuesday that it has raised $4 million in seed funding and it will use the money to support the launch of its saliva-based molecular test for oral cancers.2025-06-17View More
-
-
-

-
Original from: businesswire ¡¤ QIAGEN to create a multimodal panel using next-generation sequencing (NGS) technology for detecting clinically relevant gene alterations in hematological malignancies ..2025-06-17View More
-
-
-

-
Gene Solutions and Topgen Biomedical Technology said Thursday that they have entered a partnership under which Shaoxing, China-based Topgen will offer various genomic tests developed by Gene Solutions for cancer early detection and monitoring.2025-06-13View More
-
-
-

-
Thermo Fisher Scientific (TMO.N), opens new tab plans to sell its diagnostics unit for about $4 billion in an attempt to offload some of its low-growth assets, the Financial Times reported on Thursday, citing people familiar with the matter.2025-06-13View More
-
-
-

-
MGI Tech Co., Ltd. (¡°MGI¡±), a company dedicated to developing core tools and technologies that drive innovation in life sciences, and Asia Pathogen Genomics Initiative (¡°Asia PGI¡±) have recently announced collaboration aimed at enhancing pathogen genomics sequencing efforts across Asia. This collaboration underscores a shared commitment to supporting public health initiatives through advanced life science technology.2025-06-12View More
-
-
-

-
Paige, a leader in next-generation AI technology, and the Breast International Group (BIG), a leading international non-profit organization dedicated to breast cancer research, today announced a new partnership to conduct collaborative research aimed at improving breast cancer diagnosis, treatment, and patient outcomes.2025-06-11View More
-
-
-

-
Quest Diagnostics (NYSE: DGX), a leading provider of diagnostic information services, today announced a collaboration with The University of Texas MD Anderson Cancer Center (MD Anderson) designed to improve the assessment of elevated risk of cancer in individuals who would benefit from medically appropriate cancer screenings.2025-06-11View More
-
-
-

-
Perspectives on the IVD Market in China 2025 We are pleased to invite you to our upcoming webinar, Perspectives on the IVD Market in China 2025, tailored for overseas IVD manufacturers seeking to better ..2025-06-10View More
-
-
-

-
As metabolic dysfunction-associated steatohepatitis (MASH) emerges as one of the most dangerous and underdiagnosed drivers of liver failure¡ªaffecting more than 250 million people worldwide¡ªEchosens, the leader in non-invasive liver diagnostics, and Boehringer Ingelheim, a global biopharmaceutical company, today announced an expansion of their long-standing partnership to change the trajectory of the disease by moving beyond clinical trials to focus on early detection, diagnosis, and access to care.2025-06-10View More
-
-
-

-
Diasorin (FTSE MIB: DIA) today announced it has received 510(k) clearance from the U.S. Food and Drug Administration (FDA) for the LIAISON PLEX® Gram-Positive Blood Culture Assay, the final syndromic blood culture panel for the microbiological diagnosis of bloodstream infections on the LIAISON PLEX®.2025-06-10View More
-
-
-

-
2. Dry Chemical Technology The biggest difference between ¡°dry chemistry¡± and traditional ¡°wet chemistry¡± (i.e., solution chemistry) is the media involved in the chemical reactions. In this method, the liqui..2025-06-10View More
-
-
-

-
Thermo Fisher and Biomobio Sign Strategic Agreement in Spatial Imaging Thermo Fisher Scientific and Biomobio have successfully concluded a strategic cooperation agreement for the EVOS S1000..2025-06-09View More
-
-
-

-
New data published in JAMA Internal Medicine on Friday from MD Anderson Cancer Center researchers found that mail-in self-collection kits for human papillomavirus testing more than doubled cervical cancer screening participation among never- and under-screened patients.2025-06-09View More
-
-
-

-
Burning Rock Biotech Limited (NASDAQ: BNR, the ¡°Company¡± or ¡°Burning Rock¡±), a company focused on the application of next generation sequencing (NGS) technology in the field of precision oncology, today reported financial results for the three months ended March 31, 2025.2025-06-09View More
-
-
-

-
Fujirebio, a leading innovator in in-vitro diagnostics, today announced a collaboration with Stanford Medicine (Location: Palo Alto, California, USA) to advance research and innovation in the field of infectious disease testing. This collaboration aims to accelerate the adoption of ultrasensitive immunoassays that incorporate single-molecule counting technology developed by Fujirebio¡¯s Silicon Valley wholly-owned subsidiary, Fluxus, Inc. Greater test sensitivity can better inform treatment decisions in the clinic, as well as accelerate studies towards therapeutics and preventive strategies against infectious disease threats worldwide.2025-06-09View More
-
-
-

-
Randox Laboratories announced that the U.S. Food and Drug Administration (FDA) has approved the De Novo application for the company¡¯s first Companion Diagnostic (CDx). This is a major achievement for Randox following over 3 years of innovative development and collaboration with Novo Nordisk. The Companion Diagnostic was developed as a new option for determining the amount of medication a patient with hemophilia has received.2025-06-09View More
-
-
-

-
Cepheid today announced that Health Canada has issued Cepheid a medical device licence for Xpert® HIV-1 Viral Load XC, a next-generation extended-coverage (XC) test intended to aid in assessing HIV viral load levels, which are used to monitor effectiveness of antiretroviral treatment.2025-06-08View More
-
-
-
-
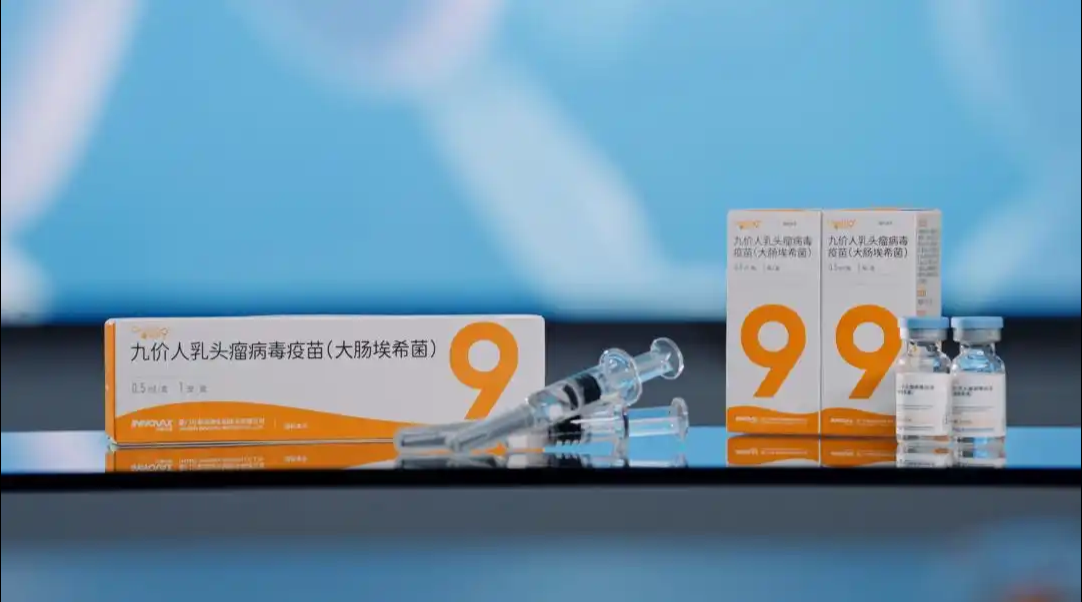
China's drug regulator has approved the country's first domestically developed 9-valent human papillomavirus (HPV) vaccine, ending over a decade of foreign dominance in the market.2025-06-08View More
-

- CAIVD WeChat
Subscription Account

- CAIVD WeChat
Channels
China Association of In-vitro Diagnostics
Part of the information in our website is from the internet.
If by any chance it violates your rights, please contact us.

