
BioPerfectus Successfully Developed COVID-19 Test Kits for B.1.617 Variant in India
2021/5/12 17:53:05 Views£º1467
The outbreak of the epidemic in India has caused continuous global attention. As of May 10, the number of new confirmed cases of COVID-19 in India exceeded 300,000 in a single day for 19 consecutive days, with a total of 22,662,575 confirmed cases and 246,116 deaths.
In order to cope with the impact of B.1.617 variant in India on the global epidemic prevention and control, the R&D team of Jiangsu BioPerfectus Yechnologies Co., Ltd quickly developed targeted test kits.

On May 11, BioPerfectus officially announced that their COVID-19 test kit for B.1.617 has been successfully developed. The product can detect three mutations based on the difference in CT value between mutant and wild-type response (∆Ct).
B.1.617 variant has become the main variant spreading locally in parts of India. Researchers sequenced the virus genome in samples from Maharashtra and found a unique combination of three mutations. It shows that COVID-19 is constantly evolving to escape the human immune system. B.1.617 variant carries three mutations in the S gene: L452R, E484Q, and P681R, which may lead to immune escape and enhanced its infectivity.
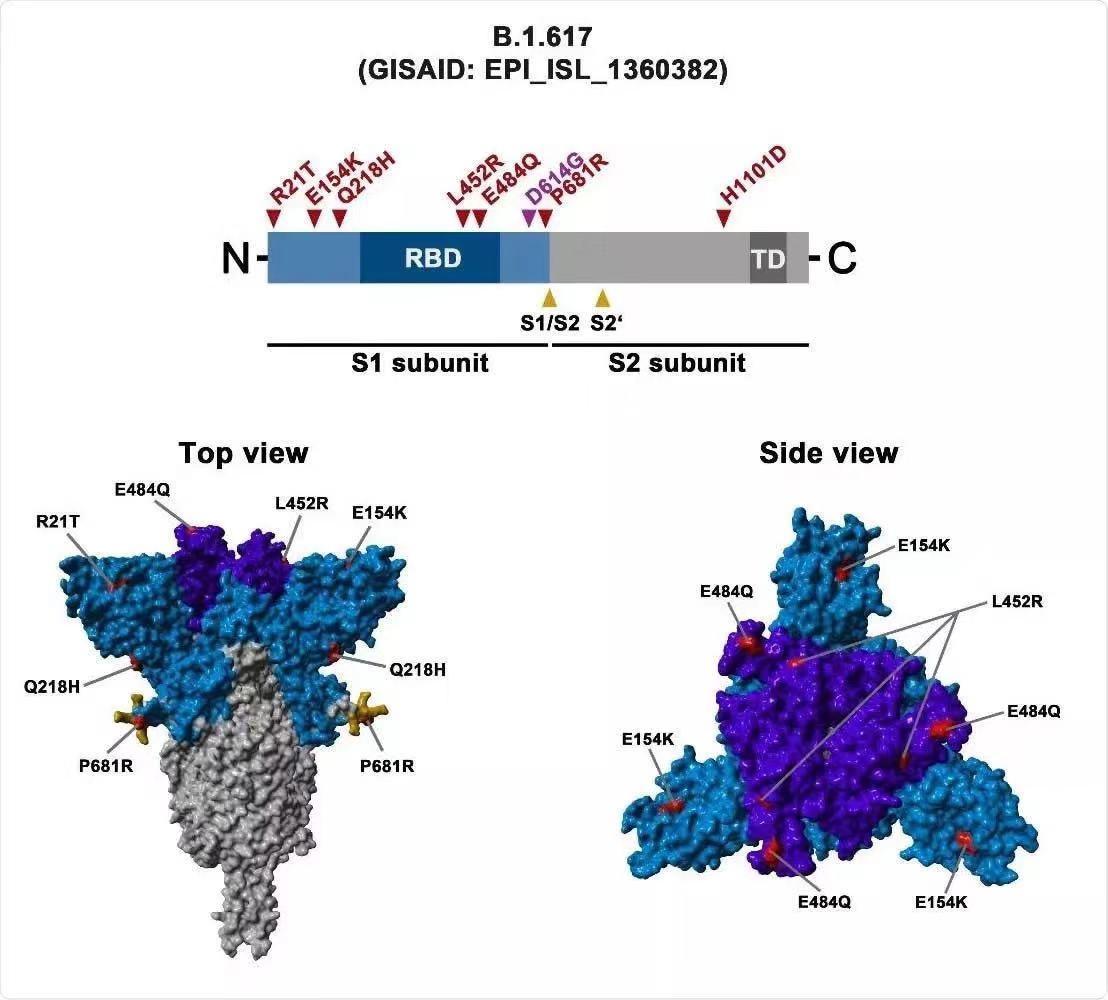

According to the latest report, the variant B.1.617 has spread to at least 20 countries and regions around the world. The World Health Organization (WHO) lists B.1.617 as a ¡°global concern variant¡± and needs to strengthen tracking and analysis. B.1.617 variant is the fourth variant designated by WHO as a global concern variant, the other three are previously discovered in the UK, South Africa and Brazil respectively.
It is worth mentioning that existing vaccines are still effective in preventing infection and deaths caused by B.1.617 according to current data.

- CAIVD WeChat
Subscription Account

- CAIVD WeChat
Channels
China Association of In-vitro Diagnostics
Part of the information in our website is from the internet.
If by any chance it violates your rights, please contact us.

