
Easy Diagnosis Authorized by PEI for COVID-19 (SARS-CoV-2) Antigen Test Kit
2021/3/11 16:52:21 Views£º1562
Recently, COVID-19 (SARS-CoV-2) Antigen Test Kit independently developed by Wuhan Easy Diagnosis passed the performance verification of Paul-Ehrlich-Institut in Germany.

PEI Authorization
Paul-Ehrlich-Institut is a research institution and medical regulatory agency of the German Federation. It is currently affiliated to the Bundesministerium f¨¹r Gesundheit (BMG). It has independent functions such as biological product inspection, clinical trial approval, product marketing approval, and batch issuance. At the same time, it is also responsible for providing scientific advice to different organizations, especially for the drafting and revision of relevant regulations of some EU countries, EU and international committees. It also provides professional advice for the German government, local agencies and parliament, and provides relevant information to patients and consumers.
The authorization of Easy Diagnosis antigen test kit fully proves its excellent performance. The current global epidemic situation is still severe, and Easy Diagnosis will continue contributing to the fight against COVID-19 epidemic.
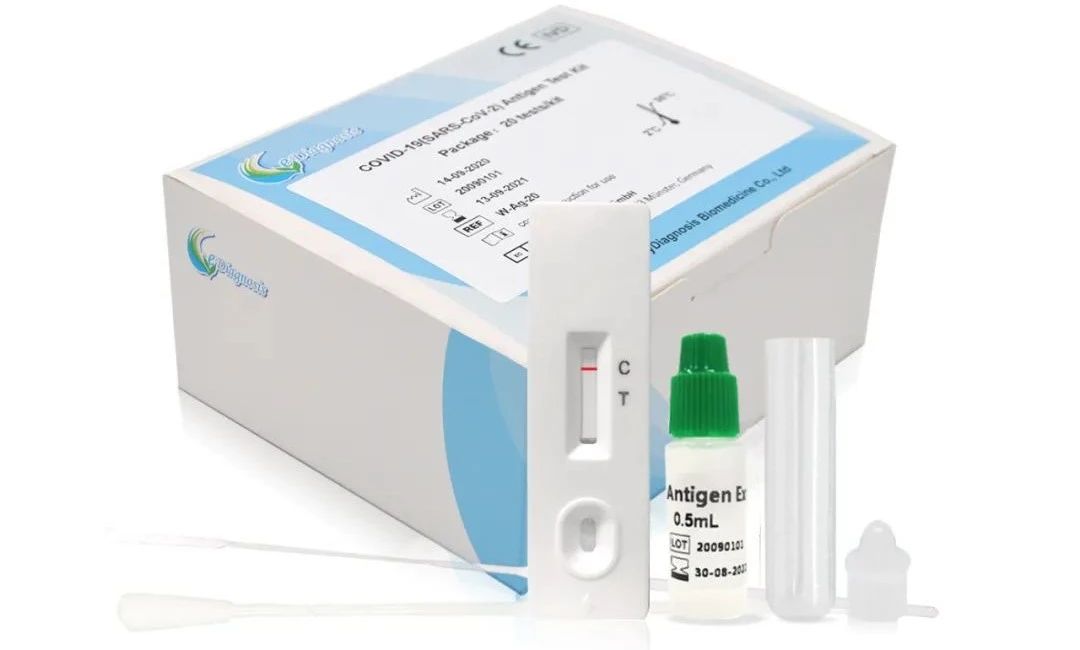
COVID-19 (SARS-CoV-2) Antigen Test Kit
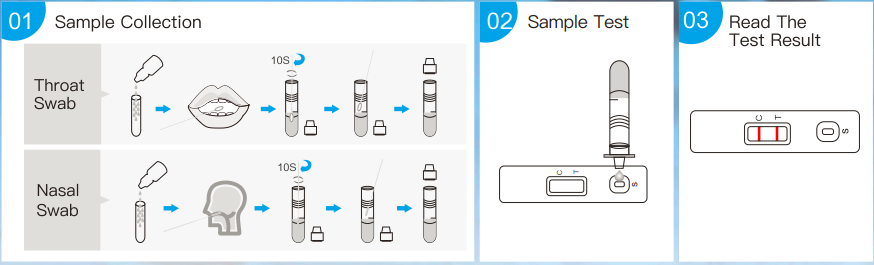
Sample Collection
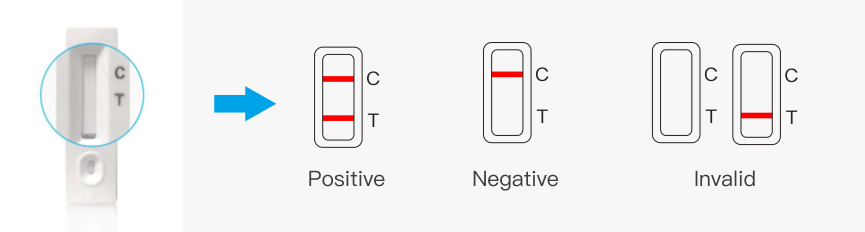
Interpretation of Result
At present, there are three main methods for detection of COVID-19. In terms of detection speed and usage scenarios, nucleic acid test is the gold standard for pathogen detection, which can detect infected persons in the first time. However, nucleic acid test requires high in detection equipment and experimental environment. The experiment takes a long time (at least 1.5 hours) for results. Thus, it is more suitable for clinical diagnosis than public initial screening. Antigen and antibody test do not require professional equipment and personnel, and only take about 15 minutes for results. Hence antigen and antibody test obtained are suitable for point-of-care testing and large-scale screening.
COVID-19 (SARS-CoV-2) Antigen Test can shorten the window period for COVDI-19 detection, and can be used for early phase of the disease (1-5 days after symptoms appear, or even during the incubation period). It is more effective for rapid testing of suspected cases of large-scale COVID-19 infection and concentrated outbreaks. In view of the more severe and complex epidemic situation in the world, many international organizations, such as WHO and FIND, are also vigorously promoting antigen test.

- CAIVD WeChat
Subscription Account

- CAIVD WeChat
Channels
China Association of In-vitro Diagnostics
Part of the information in our website is from the internet.
If by any chance it violates your rights, please contact us.

