
The First CRISPR COVID-19 Reagent Approved
2020/12/4 9:39:35 Views£ļ1287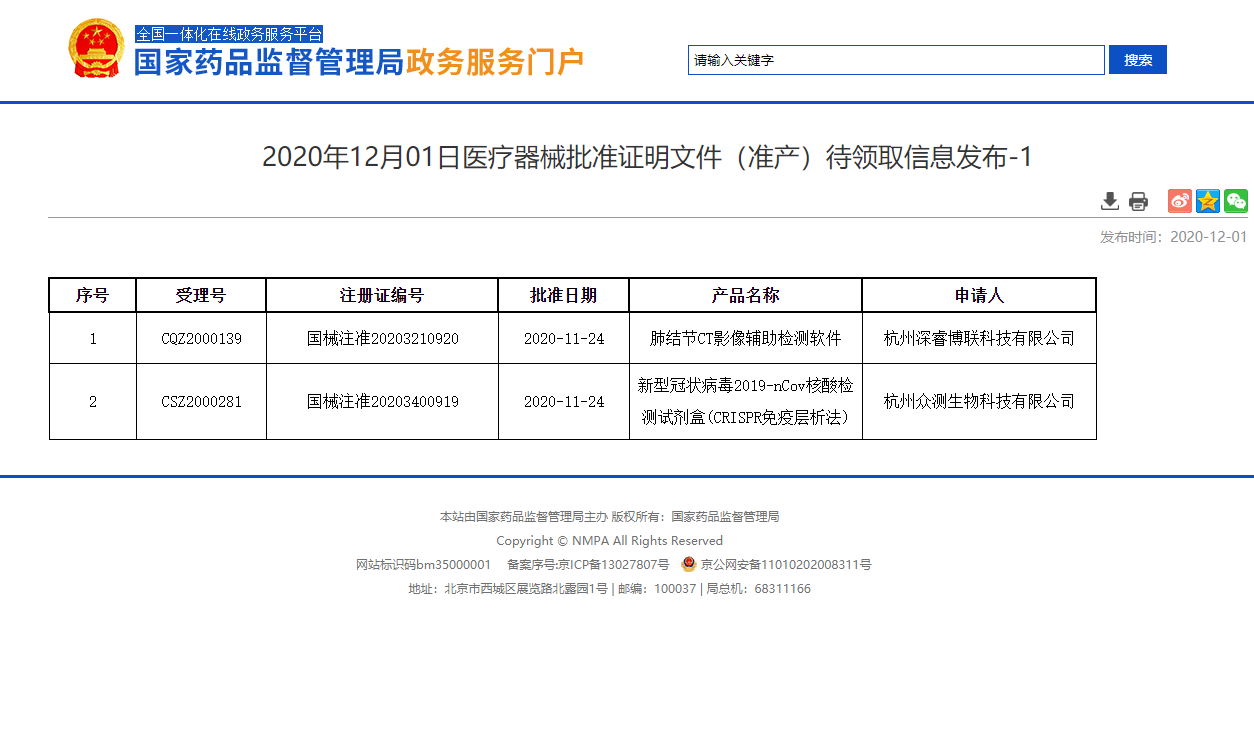
According to the info. released by NMPA, the certificate of the new type of medical equipment of 2019-nCov nucleic acid reagent kit (CRISPR with immunochromatography method) which was published on Dec. 1st researched by Hangzhou ZC Bio-Sci & Tech Co. Ltd. ("ZC Bio") has been approved.
About CRISPR
CRISPR is the hottest technology in 21 century, CRISPR once was developed accordance with the self-protective mechanisms of archaebacteria utilizing the Cas related protein editing to excision the non-self-gene to damage the invader. In recent years, the gene editing skill of CRISPR/Cas9 increasingly mature and being used on human diseases` diagnosis. The discovery of Cas12, Cas13 and Cas14 made the CRISPR application came true. The 3 of element is ubiquitous on the 2nd domain of enzyme activity. After the protein combines and excision the special nucleotide sequence correctly, the domain can be activated and cut the reported molecular then to realize the transformation from the quarantine sequence signal to detectable signal.
On April, 2017, Zhang Feng team published an article on SCIENCE expressed a method to combine the Cas13a with isothermal amplification establishing a diagnostic method of CRISPR-Dx based on CRISPR to supply a speed DNA or RNA detection with Sensitivity and single-base mismatch specificity. The research utilized the Specific High-Sensitivity Enzymatic Reporter UnLOCKing, SHERLOCK based on the cas13a molecular testing platform for the specific bacterial strain of Zika virus & dengue virus, to distinguish the pathogenic bacterium, human genotype DNA and to recognize the mutation of cell-free tumor DNA.

- CAIVD WeChat
Subscription Account

- CAIVD WeChat
Channels
China Association of In-vitro Diagnostics
Part of the information in our website is from the internet.
If by any chance it violates your rights, please contact us.

