
The Development of Companion Diagnostics In the Chinese Market
2020/11/26 11:09:57 Views£ļ1234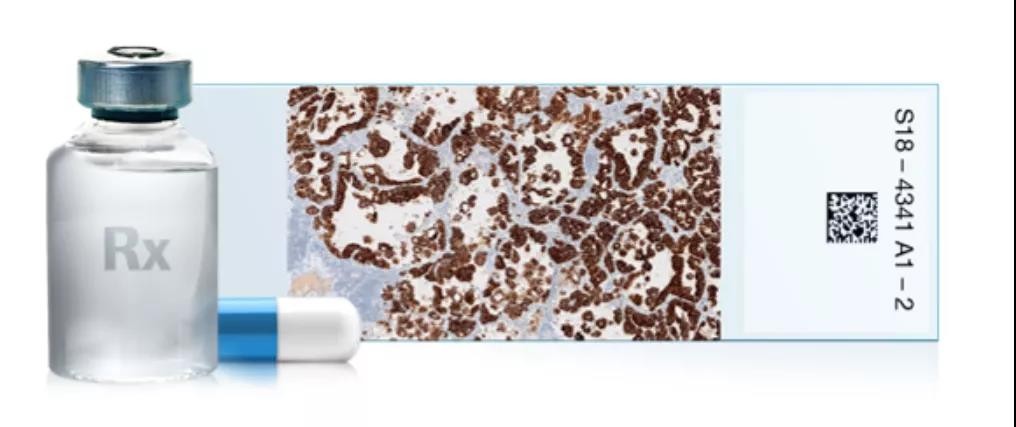
VENTANA ALK for lung cancer detection (D5F3)
Credit: Roche Diagnostics
Companion Diagnostics (CDx) is a medical product that belongs to the category of in vitro diagnostics. It can evaluate important information such as the safety and using effect of corresponding drugs or biological products. The companion diagnostic test could help medical staff judge whether a particular treatment is beneficial to the patient, thereby adjusting the treatment plan or medication plan.
The application of companion diagnostics mainly includes three aspects: one is to determine the patients who are most likely to benefit from a certain treatment; the other is to identify patients who may increase the risk of serious side effects due to the use of a specific treatment; the third is to monitor the response of specific treatment in order to improve safety or effectiveness by adjusting treatment.
One of the fastest growing segments of the IVD market
As one of the fastest growing segments of the in vitro diagnostics market, companion diagnostics is expected that its share in the in vitro diagnostics market will reach 14% in 2021. In recent years, with the support of various favorable policies, the Chinese companion diagnostics industry has entered a rapid growth phase. A pattern of diversified technology platforms and products is forming. A quite amount of companies engaged in companion diagnostics have rapidly developed and grown, innovative products continue emerge, and the market scale has expanded year by year.
Among them, a number of outstanding biomedical companies such as Amoy Dx, BGI Group, Zeesan Biotech, Tellgen, Burning Rock Dx, Geneseeq, etc., all have many self-developed genetic testing kits, and their products are exported in worldwide range.
In 2012, the Chinese companion diagnostics market was only 73 million dollars, and by 2018 it had reached 298 million dollars. It is estimated that it will exceed 741 million dollars in 2021, with a compound growth rate of 28%, which is higher than the global average.
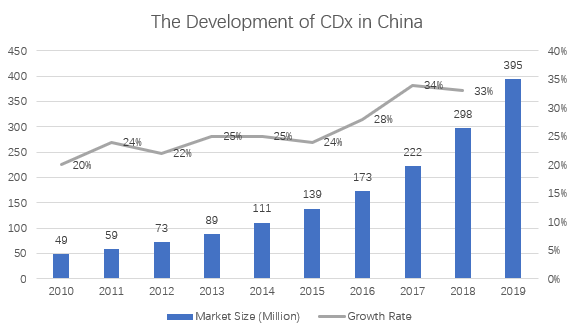
Source: cnpharm
Market distribution of companion diagnostic products
At present, companion diagnostic products are mainly developed in the form of a "drug-diagnosis" (Rx-Dx) combination. In other words, companion diagnostics and drugs are developed and taken treatment trials at the same time. From the analysis of indications, companion diagnostic products can be involved in tumors, cardiovascular and cerebrovascular diseases, inflammation, viral infections and other fields. In the oncology field, companion diagnostic products account for the largest proportion in the whole industry. At present, companion diagnostic kits for a variety of cancers have been developed and approved for market.
As of March 17, 2020, a total of 179 disease-related genetic testing kits in China have been approved by the National Medical Products Administration, of which 20.67% are concentrated in the field of tumor treatment. The other three main areas are anemia, viral infection and deafness treatment respectively. At present, the Chinese tumor gene detection kits use 4 technology platforms, mainly PCR, followed by FISH and gene chip methods.
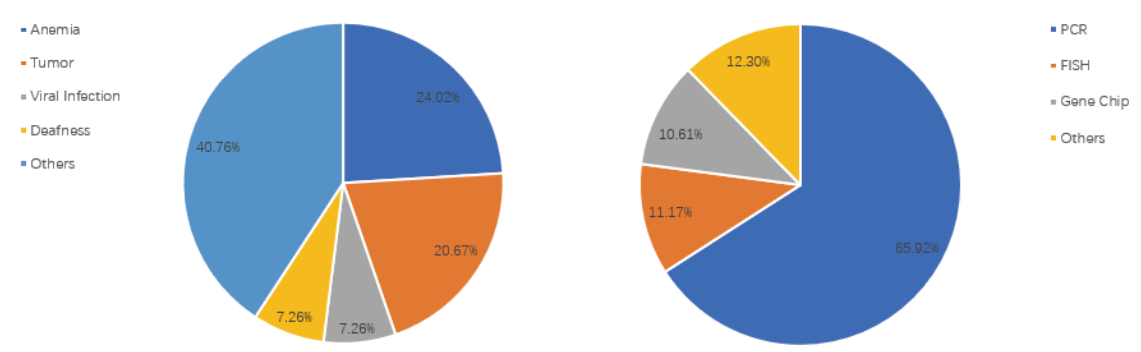
The Chinese Genetic Tests Technology Platform & Disease Distribution
Source: cnpharm

- CAIVD WeChat
Subscription Account

- CAIVD WeChat
Channels
China Association of In-vitro Diagnostics
Part of the information in our website is from the internet.
If by any chance it violates your rights, please contact us.

