
The Chinese COVID-19 Tests were Included in WHO EUL(Aug.-Sept., 2020)
2020/10/12 17:39:16 Views£º1320
According to real-time statistics from Worldometer, as of 6:30 am October 10(Beijing time), the world has recorded 3,7059,347 confirmed COVID-19 cases, 1,071,345 deaths, and more than 10,000 confirmed cases in 105 countries.
The global epidemic continues to spread. In America, the Centers for Disease Control and Prevention predicts 230,000 deaths in the United States by the end of October. A school in Brazil was closed again just three days after it reopened; in the European region, many countries have set a new high in the number of new cases in a single day in the recent rebound of the epidemic; in the African region, affected by the epidemic this year, the African economy is expected to shrink by 3.3%, reversing the 10-year economic growth trend;In Asian region, the Ministry of Health of India said that the country has the largest number of cured cases in the world, and Japan has announced a US$130 million investment in the global supply of COVID-19 vaccine.
From August to September 2020, the COVID-19 testing products of 5 companies around the world are included in the World Health Organization (WHO) Emergency Use Listing (the full English name is "Emergency Use Listing", "EUL" for short), of which 4 are Chinese in vitro diagnostics. The companies are Tellgen Corporation, Shanghai Fosun Long March Medical Science Co., Ltd., Ningbo Health Gene Technologies Co., Ltd., Beijing Wantai Biological Pharmacy Enterprise Co., Ltd., and another multinational company is Thermo Fisher Scientific.
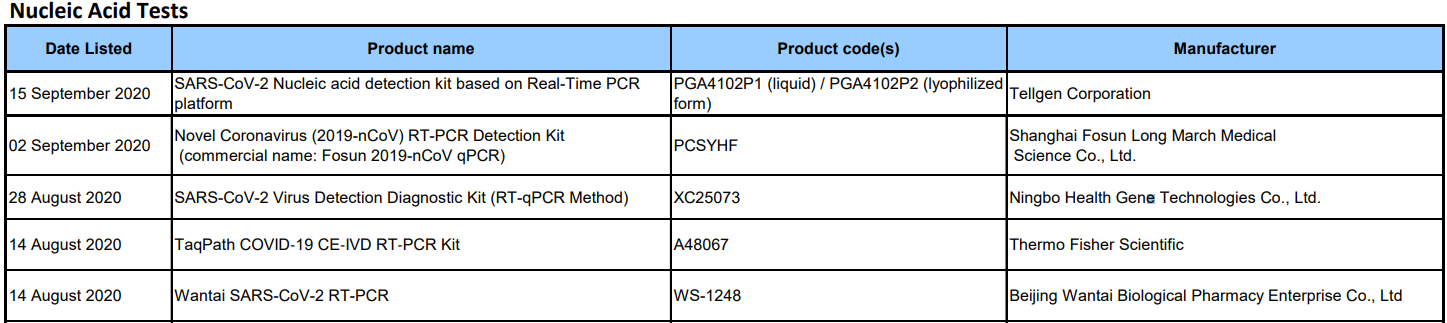
Date Listed:15 September 2020
Manufacturer£ºTellgen Corporation
Product name£ºSARS-CoV-2 Nucleic acid detection kit based on Real-Time PCR platform
Date Listed:02 September 2020¡¡
Manufacturer£ºShanghai Fosun Long March Medical Science Co., Ltd.
Product name£ºNovel Coronavirus (2019-nCoV) RT-PCR Detection Kit(commercial name: Fosun 2019-nCoV qPCR)
Date Listed:28 August 2020¡¡
Manufacturer£ºNingbo Health Gene Technologies Co., Ltd.
Product name£ºSARS-CoV-2 Virus Detection Diagnostic Kit (RT-qPCR Method)
Date Listed:14 August 2020
Manufacturer£ºThermo Fisher Scientific
Product name£ºTaqPath COVID?19 CE?IVD RT?PCR Kit
Date Listed:14 August 2020
Manufacturer£ºBeijing Wantai Biological Pharmacy Enterprise Co., Ltd.
Product name£ºWantai SARS-CoV-2 RT-PCR
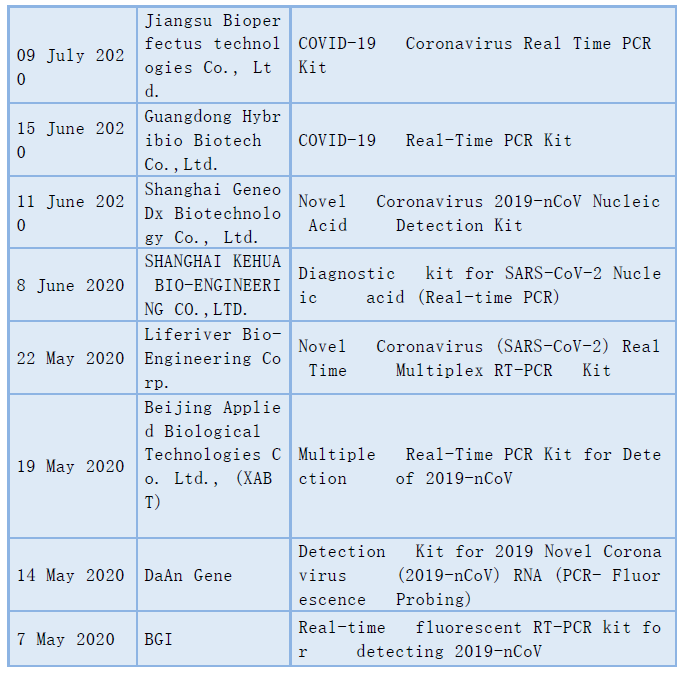
According to CACLP statistics, currently there are more than 10 chinese in vitro diagnostic manufacturers whose COVID-19 testing products have been included in the WHO EUL. In addition to the above-mentioned enterprises, there are Also Jiangsu Bioperfectus technologies Co., Ltd.,Guangdong Hybribio Biotech Co.,Ltd.,Shanghai GeneoDx Biotechnology Co., Ltd.,SHANGHAI KEHUA BIO-ENGINEERING CO.,LTD.,Liferiver Bio-Engineering Corp.,Beijing Applied Biological Technologies Co. Ltd., (XABT),DaAn Gene and BGI.

- CAIVD WeChat
Subscription Account

- CAIVD WeChat
Channels
China Association of In-vitro Diagnostics
Part of the information in our website is from the internet.
If by any chance it violates your rights, please contact us.

