
Novel Coronavirus Testing Products of 8 IVD Companies Get EUA
2020/9/27 14:25:47 Views£º1368
From July to September, domestic in vitro diagnostic companies Assure Tech. (Hangzhou) Co., Ltd., Jiangsu CoWin Biosciences Co.,Ltd., Xiamen Biotime Biotechnology Co.,Ltd.£¬Xiamen Zeesan Biotech Co.,Ltd.£¬ ZhuHai Sinochips Bioscience Co., Ltd. £¬Snibe£¬ Beijing Wantai Biological Pharmacy Enterprise Co., Ltd.£¬ Jiangsu Well Biotech Co., Ltd. have successively obtained Emergency Use Authorization(EUA) issued by US Food and Drug Administration (FDA) .
As of September 23, 2020, FDA has urgently authorized 255 novel coronavirus testing products at home and abroad. According to CACLP statistics, there are more than 19 domestic in vitro diagnostic companies.
List of domestic products that have been awarded FDA EUA from July to September 2020
(In reverse chronological order)

Jiangsu Well Biotech Co., Ltd.
Approved date: September 23, 2020
Device£ºOrawell IgM/IgG Rapid Test


Beijing Wantai Biological Pharmacy Enterprise Co., Ltd.
Approved date: September 9, 2020
Device£ºWANTAI SARS-CoV-2 RT-PCR Kit
Approved date: August 5, 2020
Device£ºWANTAI SARS-CoV-2 Ab ELISA
Approved date: July 10, 2020
Device£ºWANTAI SARS-CoV-2 Ab Rapid Test 



Snibe
Approved date: September 14, 2020
Device£ºMAGLUMI 2019-nCoV IgM/IgG


ZhuHai Sinochips Bioscience Co., Ltd.
Approved date: August 17, 2020
Device£ºCOVID-19 Nucleic Acid RT-PCR Test Kit


Xiamen Zeesan Biotech Co.,Ltd.
Approved date: July 31, 2020
Device£ºSARS-CoV-2 Test Kit (Real-time PCR)


Xiamen Biotime Biotechnology Co.,Ltd.
Approved date: July 24, 2020
Device£ºBIOTIME SARS-CoV-2 IgG/IgM Rapid Qualitative Test


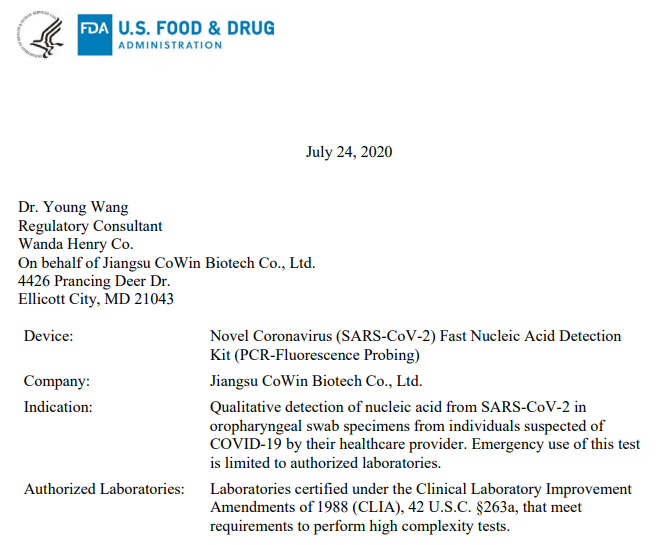
Jiangsu CoWin Biosciences Co.,Ltd.
Approved date: July 24, 2020
Device£ºNovel Coronavirus (SARS-CoV-2) Fast Nucleic Acid Detection Kit (PCR-Fluorescence Probing)

Assure Tech. (Hangzhou) Co., Ltd.
Approved date: July 6, 2020
Device£ºAssure COVID-19 IgG/IgM Rapid Test Device


- CAIVD WeChat
Subscription Account

- CAIVD WeChat
Channels
China Association of In-vitro Diagnostics
Part of the information in our website is from the internet.
If by any chance it violates your rights, please contact us.

