
China¡¯s NMPA Released The Annual Medical Device Registration Work Report in 2023 ¨C Part I
2024/2/21 12:59:39 Views£º1394
On 5 February 2024, China¡¯s National Medical Products Administration (NMPA) released the Annual Medical Device Registration Work Report in 2023.
From 1 January to 31 December 2023, China¡¯s NMPA accepted a total of 13,260 applications for the first registration, renewal of registration and change of registration of medical devices in accordance with its duties, an increase of 25.4% compared with 2022.
Acceptance of Medical Device Registration Applications
1. Overall Condition
NMPA accepted 7,106 applications for registration of Class III medical devices in China and 6,154 applications for registration of imported medical devices.
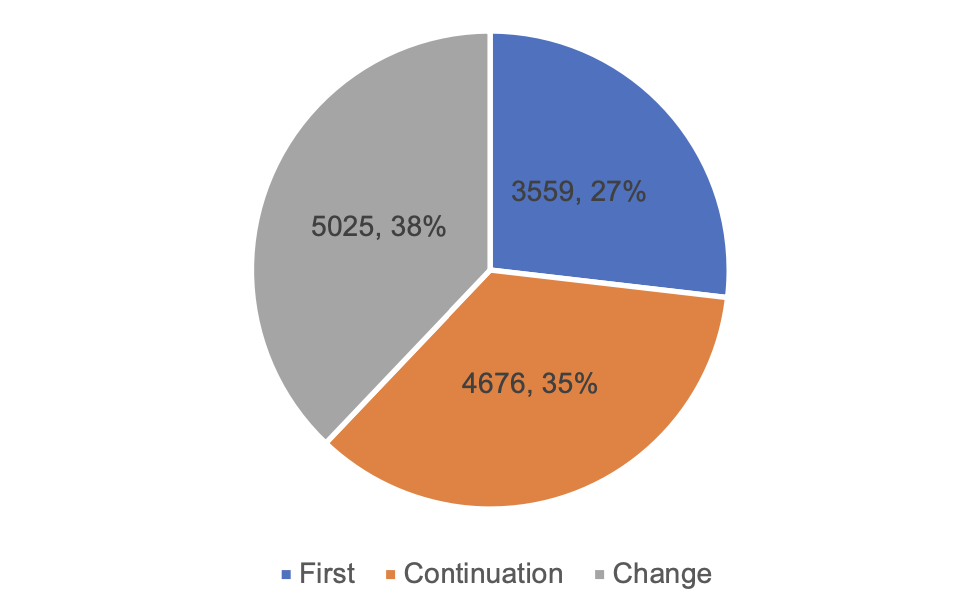
According to the registered species, 9,968 applications for registration of medical devices and 3,292 applications for registration of in vitro diagnostic reagents. Distinguished by the form of registration, the applications for first registration are 3,559, accounting for 27% of all medical device registration applications; continuation of registration application 4,676, accounting for 35%; change of registration application 5,025, accounting for 38%.
Figure 1 Proportion of Registration Forms and Quantity Accepted by NMPA
2. Specific Items
2.1 Registration of Class III Medical Devices in China
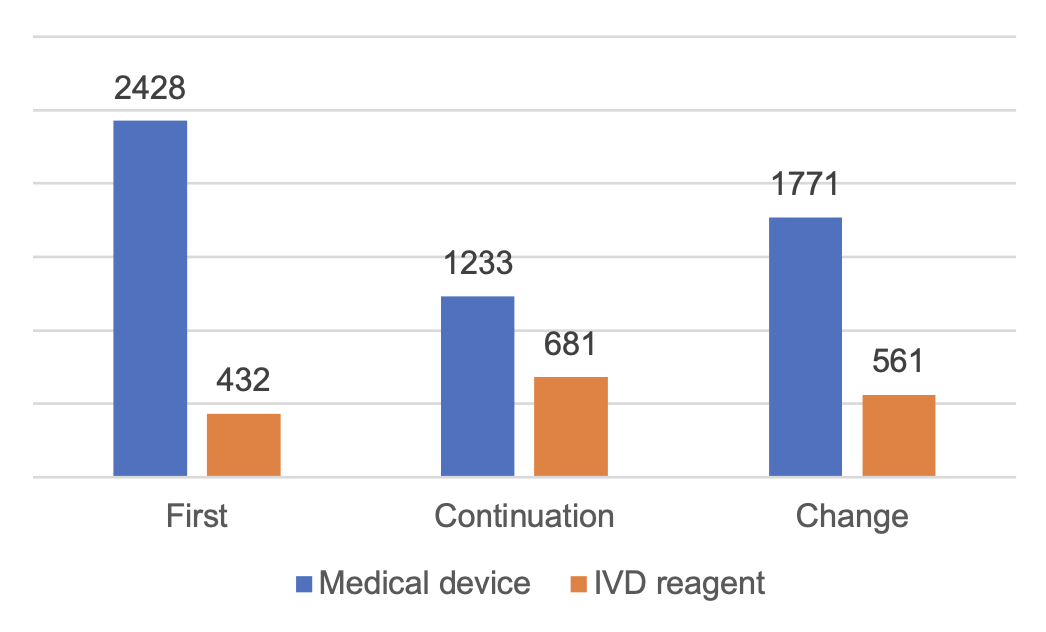
A total of 7,106 domestic Class III medical device registrations were accepted, an increase of 31% compared with 2022.
Among them, 5,432 applications for registration of medical devices and 1,674 applications for registration of in vitro diagnostic reagents.
From the registration form, the first registration of 2,860 items, accounting for 40.2% of the total number of applications for registration of Class III medical devices; the continuation of registration of 1,914 items, accounting for 26.9%; change of registration of 2,332 items, accounting for 32.8%.
Figure 2 Distribution of Class III Medical Device Registrations in China
2.2 Registration of Imported Class II Medical Devices
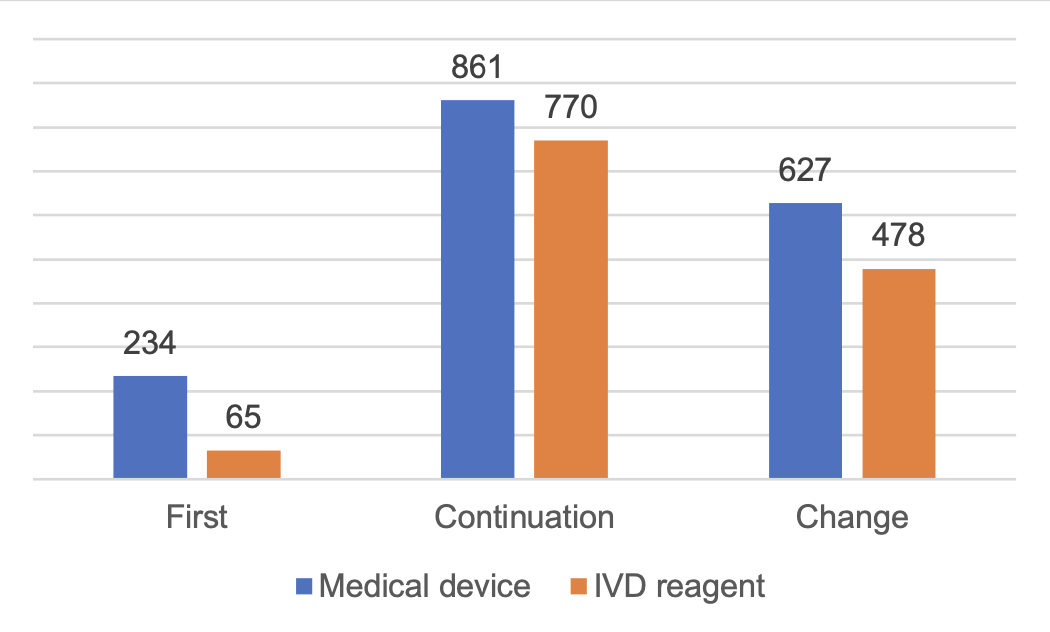
The total number of imported Class II medical devices registration is 3,036, an increase of 23.1% compared with 2022.
Among them, there are 1,723 applications for registration of medical devices and 1,313 applications for registration of in vitro diagnostic reagents.
From the registration form, the first registration of 300, accounting for 9.9% of the total number of imported Class II medical device registration applications; continuation of registration of 1,631, accounting for 53.7%; change of registration of 1,105, accounting for 36.4%.
Figure 3 Distribution of Class II Medical Device Registrations in China
2.3 Registration of Imported Class III Medical Devices
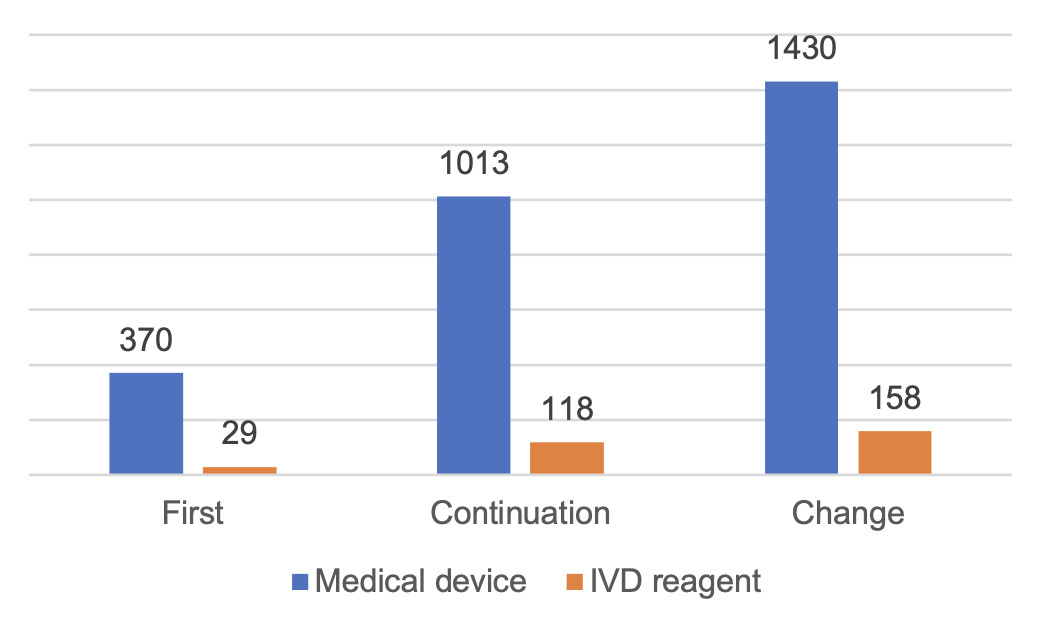
A total of 3,118 registrations for imported Class III medical devices were accepted, an increase of 16.4% compared with 2022.
Among them, 2,813 applications for registration of medical devices and 305 applications for registration of in vitro diagnostic reagents.
From the form of registration, the first registration of 399, accounting for 12.8% of the total number of applications for registration of imported Class III medical devices; continuation of registration of 1,131, accounting for 36.3%; change of registration of 1,588, accounting for 50.9%.
Figure 4 Distribution of Class III Medical Device Registrations in China

- CAIVD WeChat
Subscription Account

- CAIVD WeChat
Channels
China Association of In-vitro Diagnostics
Part of the information in our website is from the internet.
If by any chance it violates your rights, please contact us.

