
China¡¯s National Medical Products Administration released 7 IVD imported Class I medical device products ¨C do you know how to record the import of Class I medical devices?
2023/7/19 17:48:55 Views£º1064
On 5 July 2023, National Medical Products Administration announced the imported Class I medical device product in June 2023. A total of 145 product information, including 7 products in the IVD industry, were finally released.
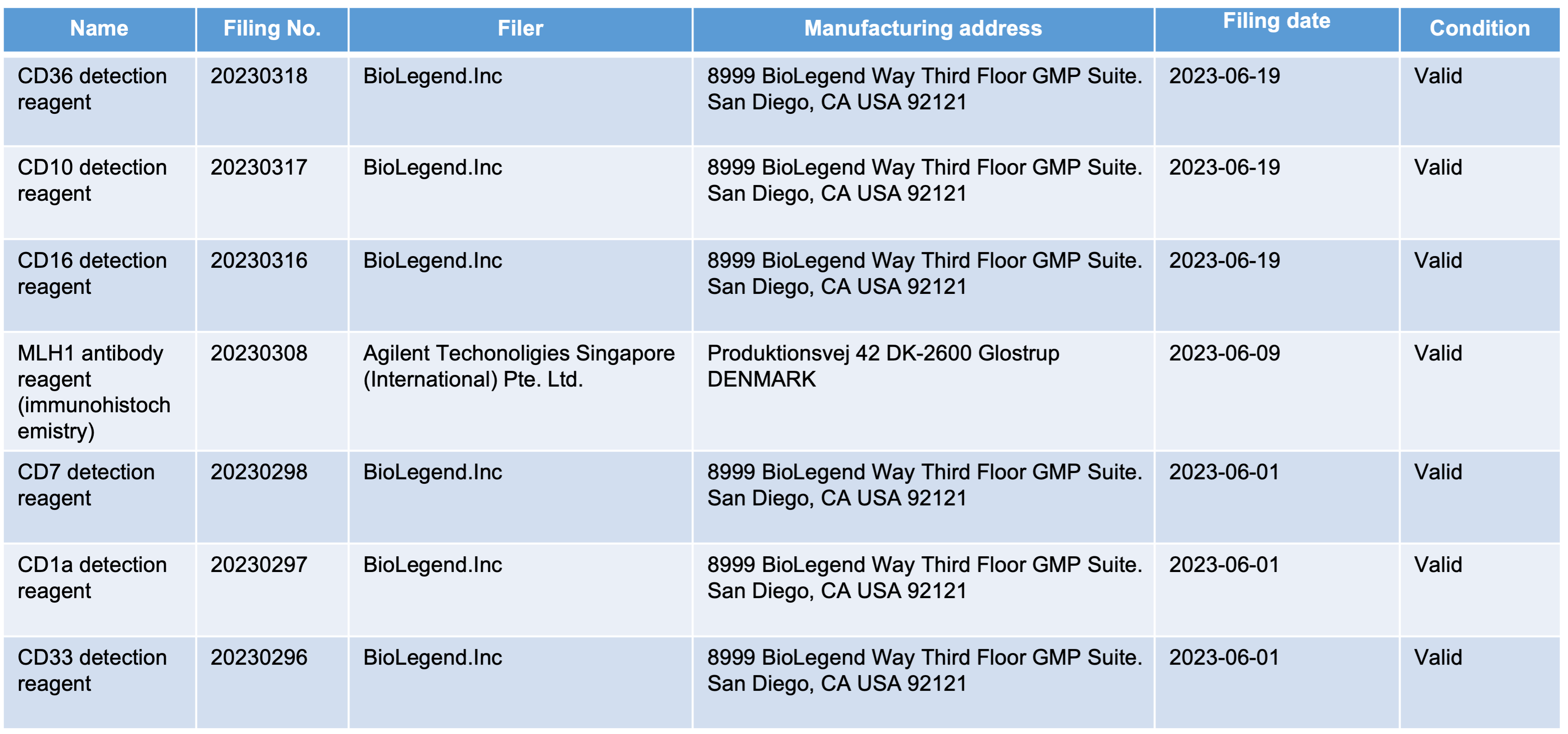
How to record the import of Class I medical devices?
The "Regulations for the Supervision and Administration of Medical Devices" clearly require that Class I medical devices implement product filing management by the filer to the National Medical Products Administration of the municipal people's government of the district where the filer is located to submit the filing information.
To export Class I medical devices to China's foreign filers, designated by the enterprise legal person in China to the National Medical Products Administration to submit the filing information and the filer's country (region) competent authorities to permit the marketing of the medical device documents. Filing information contained in the matter of change, should be changed to the original filing department for the record.
Filing process
The filer shall submit the information in accordance with the provisions of "Announcement of National Medical Products Administration on Matters Relating to the Filing of Class I Medical Devices (No. 62 of 2022)".
Filing matters are within the purview of National Medical Products Administration, the filing information is complete, in line with the formal requirements, on the spot to be filed, and provide the filing voucher filed by the filer stamped with the proprietary seal of National Medical Products Administration, will be filed in the information contained in the information table posted on the website of National Medical Products Administration to be published. Filing information is incomplete or does not meet the required form, will be informed at once the need to make corrections to all the contents of the record, the record is not filed, will inform the filer and explain the reasons.
Filing materials
According to "Announcement of National Medical Products Administration on Matters Related to the Filing of Class I Medical Devices (No. 62 of 2022)" issued in August this year, the following materials are required to apply for the filing of imported Class I medical devices (for the first time):
1. Class I medical device filing form;
2. associated documents (including ¢Ù overseas filer enterprise qualification documents, ¢Ú overseas filer registered or production place of the country (region) the competent authority of the medical device issued by the product is permitted to market and sell the documents, ¢Û overseas filer in China to designate the agent's power of attorney, the agent's letter of commitment, the agent a copy of the copy of the business license);
3. Product technical requirements;
4. Product inspection report;
5. Product instructions and minimum sales unit label design samples;
6. Manufacturing information;
7. Declaration of conformity.

- CAIVD WeChat
Subscription Account

- CAIVD WeChat
Channels
China Association of In-vitro Diagnostics
Part of the information in our website is from the internet.
If by any chance it violates your rights, please contact us.

