
Ustar Obtained 10 IVDR CE Certificate & Biotest obtained 69 EU IVDR certificate
2023/7/14 11:25:26 Views£º1845
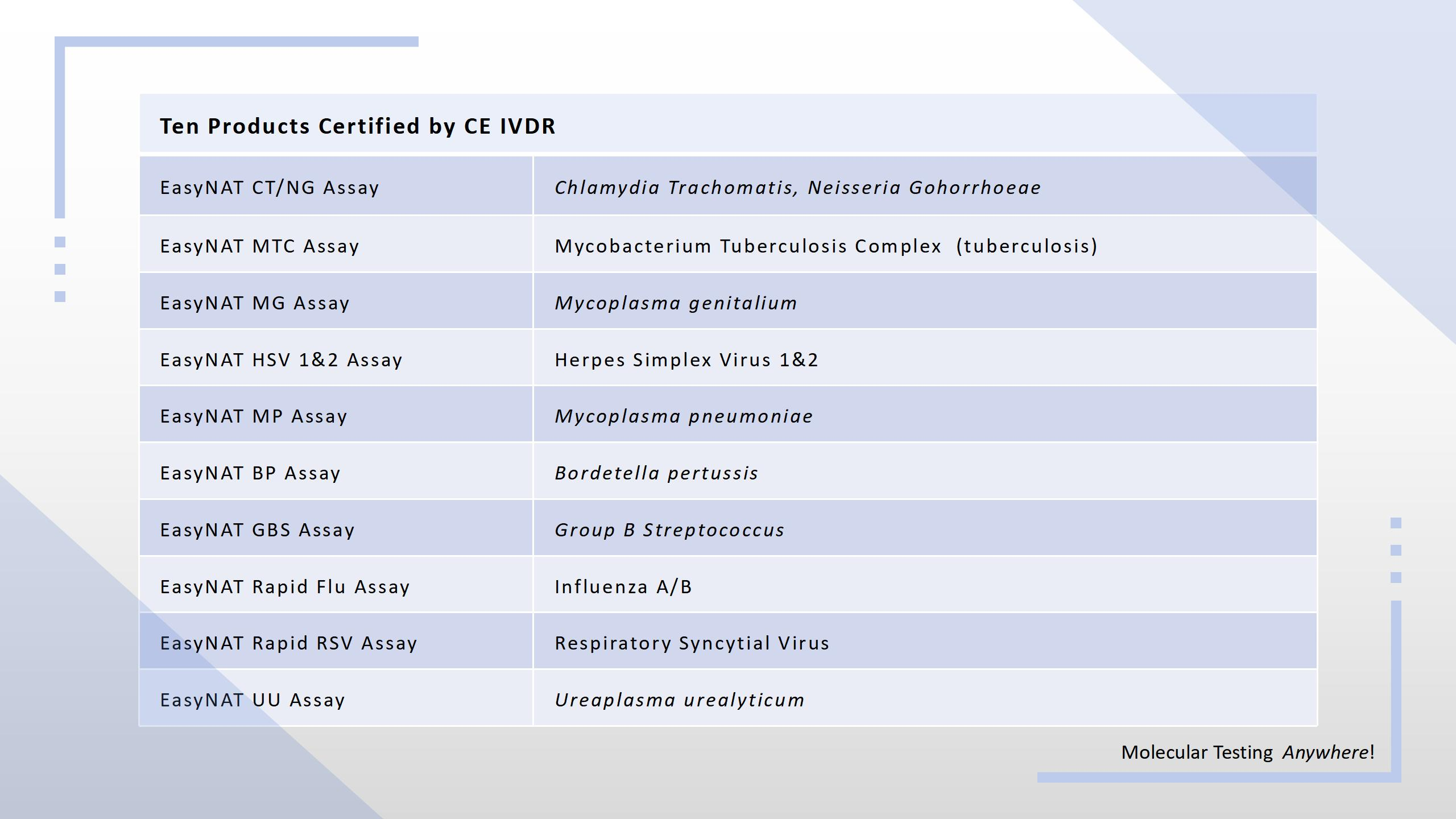
Ustar Obtains the IVDR CE Certificate for 10 Products
On July 11, Ustar received the IVDR CE certificate for 10 products from TÜV SÜD, a notified body designated under the EU's IVDR.

As one of the first IVDR-certified manufacturers for POC NAAT products in China, Ustar has made its important breakthroughs in the field of in vitro diagnostic medical devices, further consolidating its competitive advantage in the EU market.
The In Vitro Medical Devices Regulation (EU) 2017/746 (IVDR) is¡°the current regulatory basis for placing on the market, making available and putting into service in vitro diagnostic medical devices on the European market"[1]. It is intended to replace the existing EU In Vitro Diagnostic Medical Devices Directive (IVDD, Directive 98/79/EC). After the transition period, in vitro diagnostic medical devices without IVDR CE certification will no longer be able to enter the EU market. Obtaining IVDR CE certification is to ensure that the product complies with EU laws, regulations and standards, and reduces the risk of product sales in the European market, which is crucial for the sale of IVD medical devices in the European market.
The IVDR CE certificate issued to Ustar means that these ten products can be sold in Europe and other regions that accept EU certification. This greatly expands the market coverage, and provides strong support for further expanding the overseas customer base. As an IVD medical device company that meets strict safety standards and quality requirements, this certification will provide greater confidence and protection for the company's customers at home and abroad.
The first 69 products of Biotest obtained the EU IVDR certificate
Recently, Hangzhou Biotest Biotech obtained the CE certificate (IVDR) issued by TÜV SÜD, an authoritative EU notified body, under the IVDR regulation, which covers the first batch of 69 products of the company. The first batch of certified products mainly focus on the field of drug abuse testing, and also cover some items in the fields of infectious disease testing, women's health testing and tumor marker testing. The issuance of this batch of certificates makes Biotest one of the first manufacturers in China to have a more complete range of EU IVDR certified drug abuse testing products.

CE certification is a pass for products to enter the market circulation in the EU and European Free Trade Area countries, representing that the product complies with the EU laws, regulations and standards related to the product, and is a commitment of the enterprise to consumers, which can increase the trust of consumers in the product and at the same time reduce the risk of selling in the European market.
The new EU In Vitro Diagnostic Medical Devices CE Certification Regulation (IVDR, EU2017/746) was officially published and came into force on May 25, 2017 and will be implemented from May 26, 2022. Since the implementation date, the EU IVDR regulation will replace the former EU In Vitro Diagnostic Medical Devices Directive (IVDD, 98/79/EC) to regulate in vitro diagnostic medical devices in the EU market, and products that have obtained CE certification before May 26, 2022 will be gradually switched over during the transition period according to the risk level. The new IVDR regulation classifies IVD products into four categories, with risk ranked from low to high: Class A, Class B, Class C and Class D. Class A is sterile, while Class B, C and D require the EU Notification Body (Notify Body) to issue certificates before the products can enter the EU market. The new IVDR regulation aims to establish a modern and stricter regulatory framework in order to better protect the health and safety of the public and patients. Compared with the IVDD Directive, the IVDR Regulation imposes stricter requirements on manufacturers' quality systems and on the safety, efficacy and post-market surveillance of products, and requires products to meet traceability from production to endpoint.
The acquisition of the IVDR certificate for quality management system and IVDR certificates for several products is another milestone breakthrough since the company was listed on the Science and Technology Board and obtained the Medical Device Single Audit Procedure (MDSAP) certificate, which is of great significance in practicing the company's quality policy and differentiated product development strategy. The acquisition of the new IVDR certificate marks, on one hand, that Biotest has become a medical device manufacturer that meets the requirements of the new EU in vitro diagnostic medical device regulations for IVDR CE certification, laying a solid foundation for the subsequent switchover of the company's other products to EU certification. On the other hand, it is also a recognition of the hard work of all IVDR project participants in the past year, and inspires Biotest people to complete the IVDR registration of subsequent products faster and with higher quality.
The new IVDR quality management system certificate and IVDR product certification have also brought positive impact in marketing. The new IVDR certification will not only promote the sales growth of related products in the EU region, but also help the registration and certification of related products in non-EU regions. With this IVDR certification, Biotest will accelerate the improvement of the global layout of its product line and bring more convenient and instant diagnostic services to people around the world.
About Ustar
USTAR BIOTECHNOLOGIES (HANGZHOU) LTD. was established in 2005, dedicated to the research, development, manufacturing and sales of innovative point-of-care molecular diagnostic technology and products. We have established a technical platform with completely independent IP rights, and applied for over 90 international and domestic invention patents, including 45 authorized patents. Ustar keeps participating in national projects for the prevention and control of infectious diseases, and has won dozens of national grants and awards. Our nucleic acid amplification and detection analyzer was certified by National Medical Products Administration (NMPA) through the emergency approval channel in 2019; the COVID-19 nucleic acid test kit obtained NMPA registration certificate in early 2020 as the first domestic approved listed COVID-19 molecular POCT product. Ustar¡¯s POCT kits integrate nucleic acid extraction, purification, amplification and test in an enclosed cartridge to achieve a fast, accurate, simple and safe test with LOD¡Ü200 copies/mL.
Ustar's products have served nearly 3,000 medical institutions and exported to over 70 countries, actively contributing to the global prevention and control of major infectious diseases such as COVID-19 and tuberculosis. Our vision is to make molecular tests available to anyone, anywhere, as stated in our slogan, Molecular Testing Anywhere !
About Biotest
Founded in 2008, Hangzhou Biotest Biotech Co., Ltd. is a national high-tech enterprise that mainly develops and produces rapid diagnostic reagents. Our leading products include infectious disease testing, drug abuse testing, tumor marker testing, cardiac marker testing and reproductive health testing series, which are widely used in hospitals, CDCs, blood stations, drug treatment bans and third-party testing institutions at all levels. Biotest focuses on independent research and development of products and technology accumulation, and has a highly innovative and pioneering R&D team, which provides many patented technologies and innovative product development for enterprises every year. Relying on strong research and development and technological innovation capabilities, the company is in the leading position in the research of cutting-edge biological fields such as colloidal gold, latex, fluorescence and other labeling technologies, mono/polyclonal antibodies, genetic recombinant antigens, synthetic antigens and other biological raw materials technologies, as well as immunochromatographic technologies. Adhering to the principle of quality first, Biotest has obtained several quality system certifications, including ISO13485 quality system certification, NMPA quality system certification, and MDSAP quality system certification recognized by many countries, including the United States, Canada, Brazil, Japan, Australia, etc.

- CAIVD WeChat
Subscription Account

- CAIVD WeChat
Channels
China Association of In-vitro Diagnostics
Part of the information in our website is from the internet.
If by any chance it violates your rights, please contact us.

