
Another Victory! RocGene¡¯s Archimed Mini 16 Obtains Class III Medical Device Registration Certificate
2023/2/15 13:04:22 Views£º1161
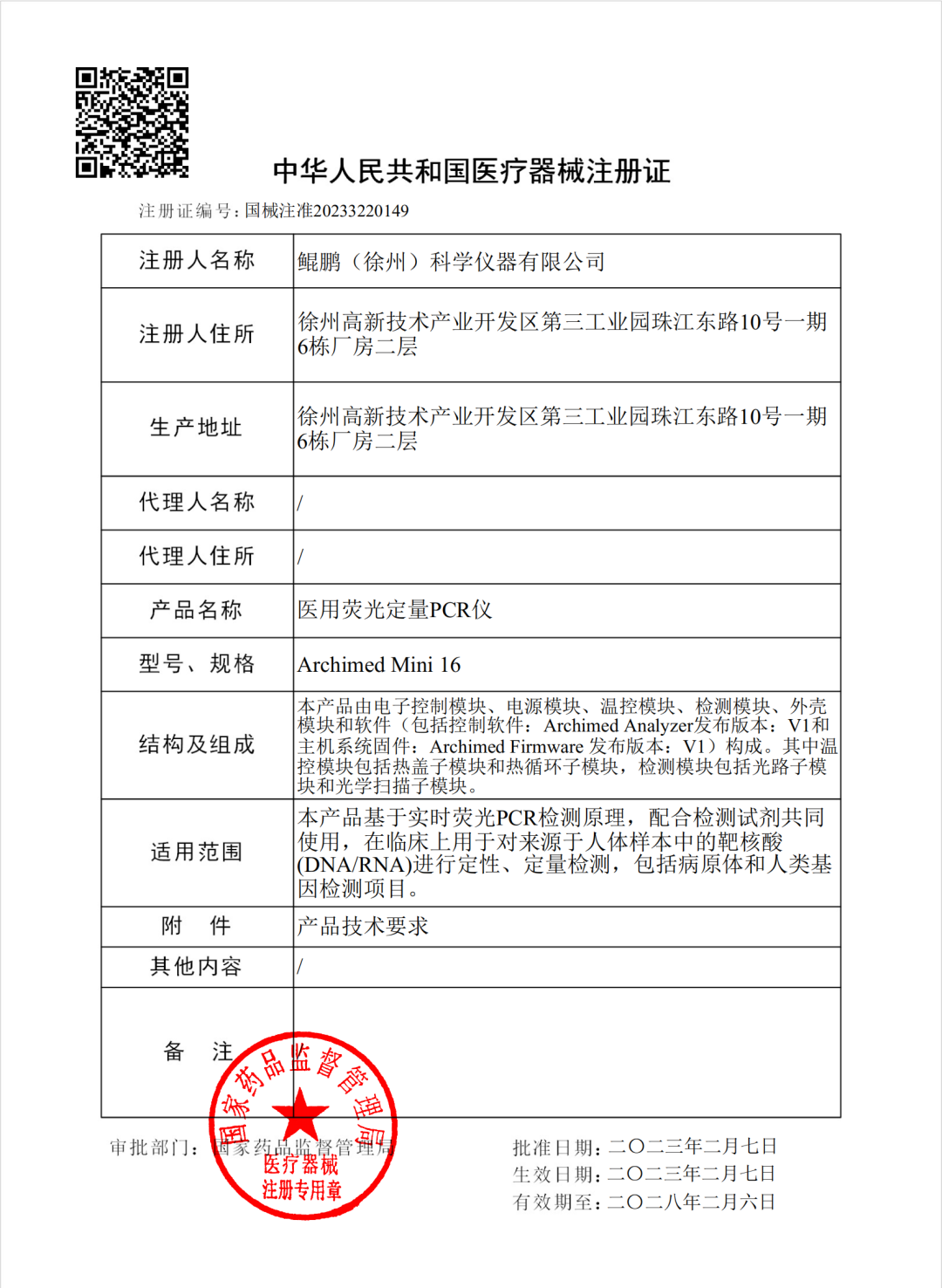
On February 7 2023, RocGene's independently developed Archimed Mini 16 Medical Fluorescence Quantitative PCR Instrument obtained the Class III Medical Device Registration Certificate approved by the National Medical Products Administration, with registration number of NMPA Approval No. 20233220149.
This is the second clinical diagnosis-related qualification obtained by RocGene' in the field of molecular diagnostic equipment after the Archimed X series medical fluorescent quantitative PCR instrument was certified. Since then, the RocGene fluorescent PCR product line has covered multi-purpose and multi-scenario solutions from high-throughput 96-well general-purpose quantitative PCR (Archimed X4/X6) to fast and portable quantitative PCR (Archimed Mini 16). It will meet the full-scenario needs of various medical and health institutions including tertiary hospitals, district and county-level hospitals, primary health service institutions, CDCs at all levels, laboratory departments, outpatient/emergency departments, and clinical departments.
Rapid acquisition of test results is an important application requirement for on-site real-time testing. In order to meet such practical needs, Archimed Mini 16 uses innovative Peltier semiconductors combined with liquid circulation heat dissipation technology to achieve ultra-fast temperature changes while ensuring uniform and precise temperature control performance, the maximum sample temperature change rate is greater than 5.4 ¡æ/ sec. Under the same reagents and operating conditions, compared with the market universal (96-well) quantitative PCR, the experimental time is reduced by at least 30%.

- CAIVD WeChat
Subscription Account

- CAIVD WeChat
Channels
China Association of In-vitro Diagnostics
Part of the information in our website is from the internet.
If by any chance it violates your rights, please contact us.

