
NMPA Approves the First Deafness Genes Detection Kit Based on Mass Spectrometry
2023/1/11 14:30:26 Views£º1561
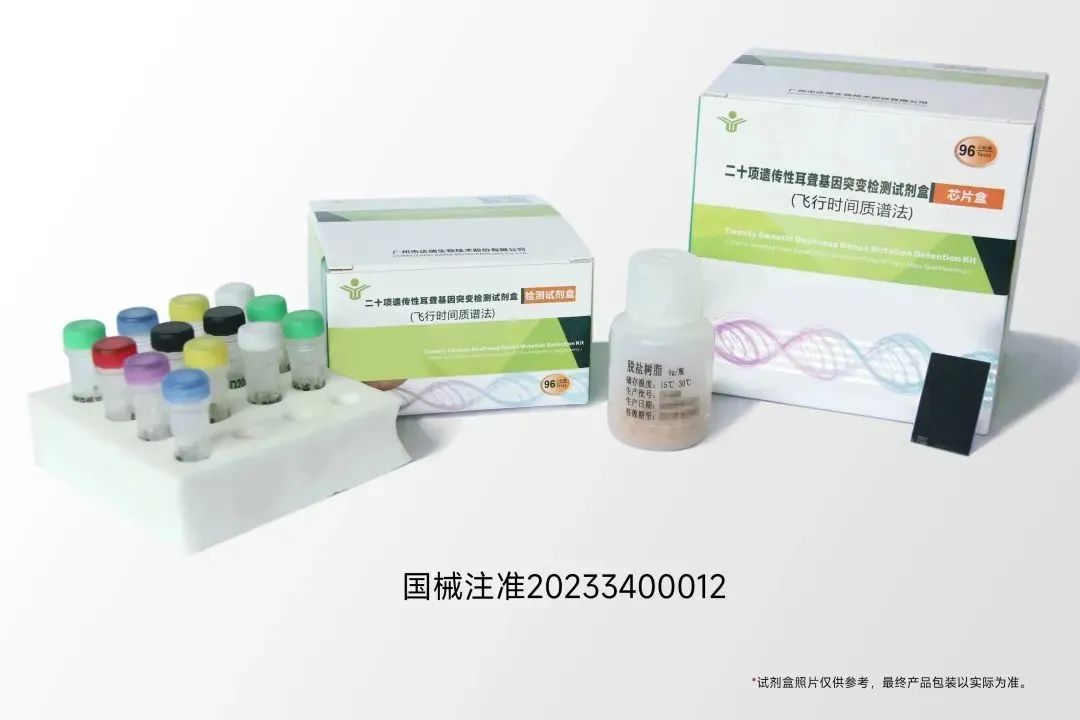
On January 5, 2023, Guangzhou Darui Biotechnology Co., Ltd., the subsidiary of Daan Gene Co., Ltd., received China National Medical Products Administration (NMPA) class III medical device approval for its ¡°Twenty Genetic Deafness Genes Mutation Detection Kit (Time of Flight Mass Spectrometry)¡±. It is the first approved deafness genes detection kit based on mass spectrometry.
Clinical Significance
In China, the incidence of newborn deafness is 1¡ë-3.47¡ë and the proportion caused by genetic factors is 50%-60%. There are clear common deafness genes and causative mutation hotspots among Chinese although many deafness-related genes exit. The carrier rate of deafness genes and causative mutation of Chinese is at least 15%, of which about 70% are from GJB2, SLC26A4, mitochondrial DNA 12SrRNA and GJB3.
Why taking genetic deafness gene detection?
(1) Diagnose patients with congenital hereditary hearing loss and intervene in the early phase combined with hearing test results.
(2) Identify patients with delayed hereditary hearing loss in the early phase and apply effective prevention and treatment to slow the hearing loss.
(3) Identify the carriers of drug-induced deafness susceptibility gene mutations and avoid hearing loss under medication guide.
(4) Identify the carriers of deafness gene mutation in the early phase when they still have normal hearing and offer accurate genetic counseling about marriage and childbirth to carriers and their family members.
(5) Reduce social and household payment through early prevention and intervention.
Product Advantages
Comprehensive coverage
A single reaction well can detect 4 genes and 20 sites, covering most of hotspots of Chinese people.
Easy to operate
The detection kit is matched with DR MassARRA automated operating system and strong data analysis software that greatly reduces the manual operation time.
High accuracy
The typing results of DR MassARRAY platform had a concordance rate of 99.99% with the results of the gold standard Sanger sequencing method.
Flexible throughput
The 96-well chip equipped with the DR MassARRAY system can be used in batches and one or two chips can be detected on the machine at a time. The detection sample throughput is flexible.
Cost-effective
Using conventional PCR reagents. No fluorescent probes are required. Multiple target detections are concentrated in as few reactions as possible, which efficiently saves time, reagents, and labor costs.
DR MassARRAY Time of Flight Mass Spectrometry

DR MassARRAY is a medium-throughput gene sequencing system developed and produced in China, specially designed for hospitals and clinical testing centers. It perfectly integrates the high sensitivity of PCR technology, the high throughput of chip technology, and the high accuracy and intelligence of mass spectrometry technology. It is the first approved mass spectrometry detection system in China that can be directly used for nucleic acid detection.

- CAIVD WeChat
Subscription Account

- CAIVD WeChat
Channels
China Association of In-vitro Diagnostics
Part of the information in our website is from the internet.
If by any chance it violates your rights, please contact us.

