
NMPA Authorizes ZJ Bio-Tech AutraMic Mini4800 Plus
2022/9/14 15:01:18 Views£ļ1497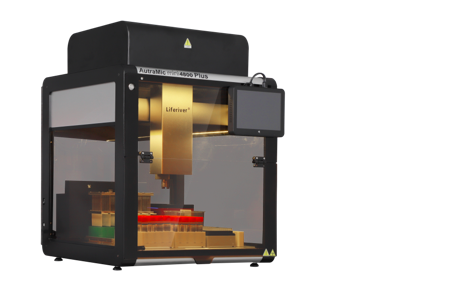
Recently, the AutraMic mini4800 Plus developed by Shanghai ZJ Bio-Tech Co.,Ltd. was approved by National Medical Products Administration. It was the smallest and more automated integrated magnetic bead method + fluorescent PCR nucleic acid detection system.
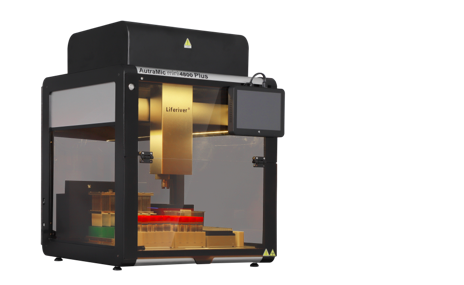
According to the introduction, AutraMic mini4800 Plus can achieve 100% nucleic acid testing "sample in, result out", integrated with a number of cutting-edge technology, has the following technical advantages:
1. Fully Automated
AutraMic mini4800 Plus can complete the entire process of nucleic acid testing, including sample tube opening and closing, pre-treatment, nano-magnetic bead extraction, PCR system construction, and fluorescent PCR amplification automatedly. With zero contact between personnel and samples during the testing process, the risk of operational errors due to personnel fatigue can be effectively avoided, alleviating the current problem of insufficient number of professional nucleic acid testing personnel.
2. Highly Efficient and Rapid Test
It is equipped with 48 PCR amplification wells and can complete 48 tubes of samples in 2 hours. If paired with the 20 mix 1 solution, at least 960 samples can be completed in 2 hours. It has flexible settings for single test throughput of 1-48 samples, which can effectively improve outpatient and emergency nucleic acid screening and review, and alleviate the outstanding contradiction of staff shortage, so that it can also quickly enhance the fully automated nucleic acid testing capacity of medical institutions, CDCs, airport ports, etc.
3. Small and Highly Secure
It highly integrates the modules of the nucleic acid testing process, covering an area of less than 0.4m2, greatly saving the tight space in the laboratory and maximising the nucleic acid testing capacity within the limited space. It is also equipped with a fully enclosed negative pressure system, UV disinfection and HEPA high efficiency filtration system, which meets the biosafety level 2 standard and fully protects the operational safety of the testers.

- CAIVD WeChat
Subscription Account

- CAIVD WeChat
Channels
China Association of In-vitro Diagnostics
Part of the information in our website is from the internet.
If by any chance it violates your rights, please contact us.

