
AutoMeloc Monkeypox nucleic acid detection kit (PCR-fluorescent probe method) obtained CE mark
2022/5/26 17:46:42 Views£ļ1122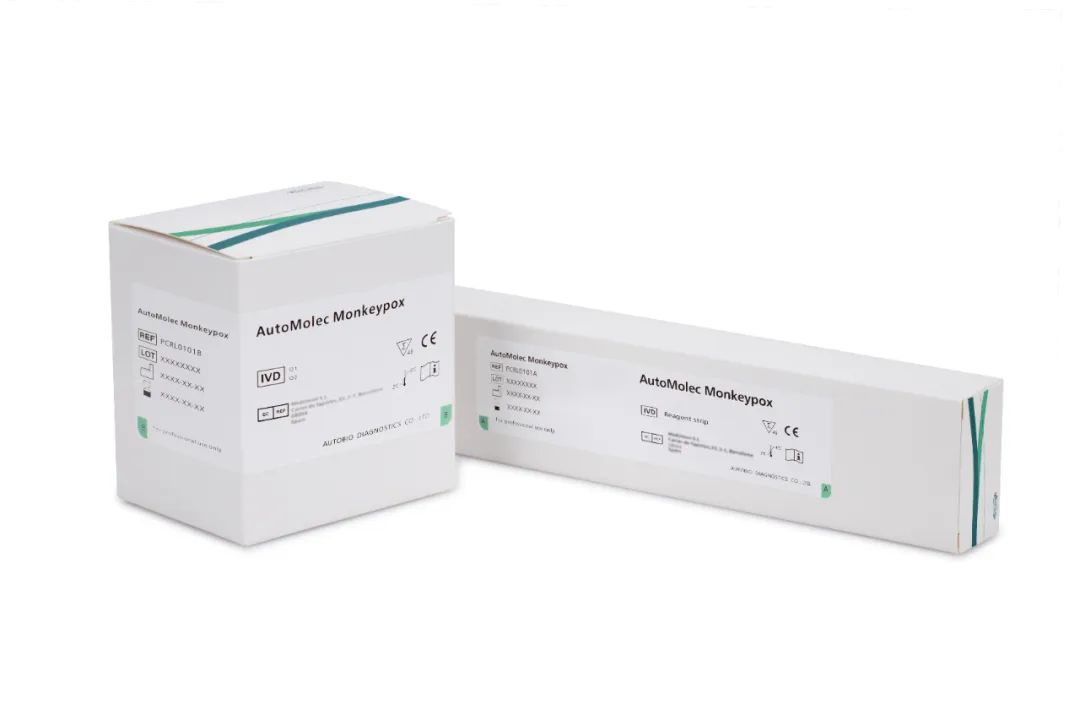
On May 23, 2022, Autobio Diagnostics Co., Ltd. announces CE mark for AutoMeloc Monkeypox nucleic acid detection kit (PCR-fluorescent probe method).
Recently, many countries have reported confirmed or suspected cases of human infection with monkeypox virus, and the World Health Organization has also issued a monkeypox outbreak warning on May 21. Autobio organized the research and development of monkeypox detection products for the first time, and successfully developed a monkeypox nucleic acid detection kit (PCR-fluorescent probe method).
AutoMeloc Monkeypox nucleic acid detection kit adopts real-time fluorescent PCR technology, which has high sensitivity and good specificity. It can accurately detect the specific gene fragments of monkeypox virus and realize early and rapid diagnosis of suspected infection. It can be used in conjunction with Autobio's in vitro molecular detection instrument for sample extraction and amplification analysis to realize random automatic detection of samples.

In vitro molecular detection instrument for sample extraction and amplification analysis
AutoMolec 3000, AutoMolec 1600

- CAIVD WeChat
Subscription Account

- CAIVD WeChat
Channels
China Association of In-vitro Diagnostics
Part of the information in our website is from the internet.
If by any chance it violates your rights, please contact us.

