


Original from The Lancet
Background
To date, no immunological data on COVID-19 heterologous vaccination schedules in humans have been reported. We assessed the immunogenicity and reactogenicity of BNT162b2 (Comirnaty, BioNTech, Mainz, Germany) administered as second dose in participants primed with ChAdOx1-S (Vaxzevria, AstraZeneca, Oxford, UK).

Methods
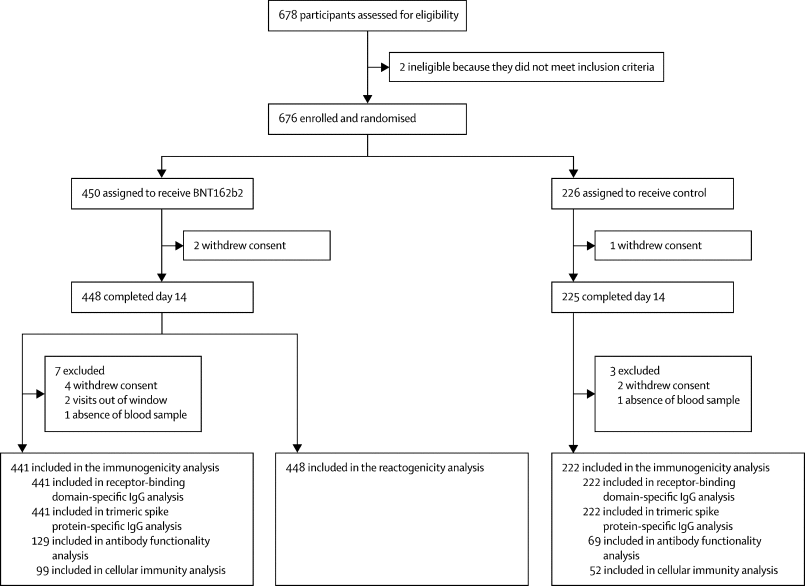
We did a phase 2, open-label, randomised, controlled trial on adults aged 18每60 years, vaccinated with a single dose of ChAdOx1-S 8每12 weeks before screening, and no history of SARS-CoV-2 infection. Participants were randomly assigned (2:1) to receive either BNT162b2 (0﹞3 mL) via a single intramuscular injection (intervention group) or continue observation (control group). The primary outcome was 14-day immunogenicity, measured by immunoassays for SARS-CoV-2 trimeric spike protein and receptor binding domain (RBD). Antibody functionality was assessed using a pseudovirus neutralisation assay, and cellular immune response using an interferon-污 immunoassay. The safety outcome was 7-day reactogenicity, measured as solicited local and systemic adverse events. The primary analysis included all participants who received at least one dose of BNT162b2 and who had at least one efficacy evaluation after baseline. The safety analysis included all participants who received BNT162b2. This study is registered with EudraCT (2021-001978-37) and ClinicalTrials.gov (NCT04860739) and is ongoing.
Findings
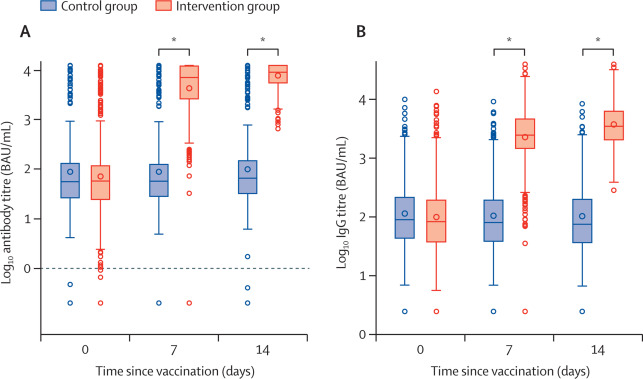
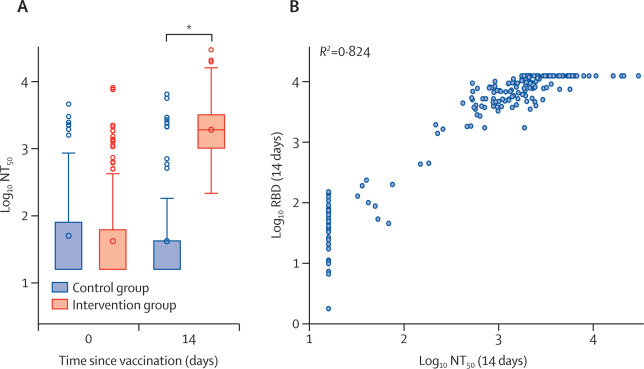
Between April 24 and 30, 2021, 676 individuals were enrolled and randomly assigned to either the intervention group (n=450) or control group (n=226) at five university hospitals in Spain (mean age 44 years [SD 9]; 382 [57%] women and 294 [43%] men). 663 (98%) participants (n=441 intervention, n=222 control) completed the study up to day 14. In the intervention group, geometric mean titres of RBD antibodies increased from 71﹞46 BAU/mL (95% CI 59﹞84每85﹞33) at baseline to 7756﹞68 BAU/mL (7371﹞53每8161﹞96) at day 14 (p<0﹞0001). IgG against trimeric spike protein increased from 98﹞40 BAU/mL (95% CI 85﹞69每112﹞99) to 3684﹞87 BAU/mL (3429﹞87每3958﹞83). The interventional:control ratio was 77﹞69 (95% CI 59﹞57每101﹞32) for RBD protein and 36﹞41 (29﹞31每45﹞23) for trimeric spike protein IgG. Reactions were mild (n=1210 [68%]) or moderate (n=530 [30%]), with injection site pain (n=395 [88%]), induration (n=159 [35%]), headache (n=199 [44%]), and myalgia (n=194 [43%]) the most commonly reported adverse events. No serious adverse events were reported.
Interpretation
BNT162b2 given as a second dose in individuals prime vaccinated with ChAdOx1-S induced a robust immune response, with an acceptable and manageable reactogenicity profile.
View source version on:https://www.thelancet.com/journals/lancet/article/PIIS0140-6736(21)01420-3/fulltext