



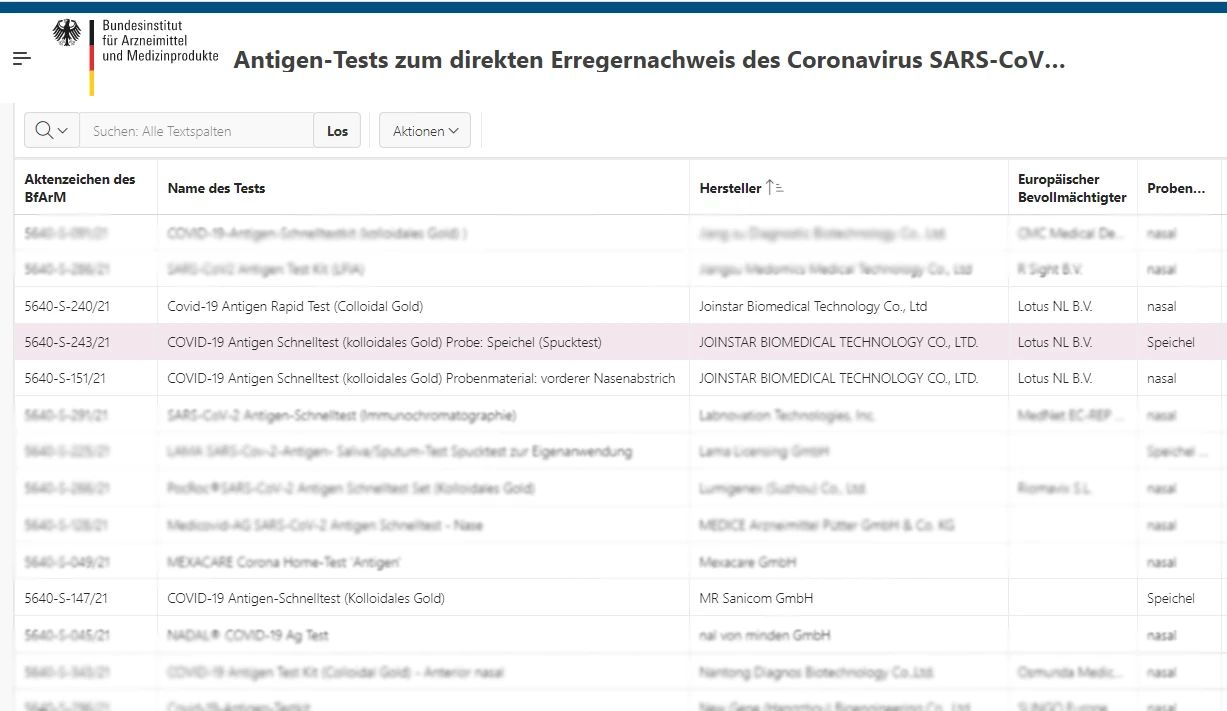
On June 21st, COVID-19 Antigen Rapid Test (Colloidal Gold) COVID-19 Antigen Schnelltest (kolloidales Gold) Probe: Speichel (Spucktest) developed by Joinstar Biomedical Technology Co., Ltd. obtained the approval of the German Federal Agency for Medicines and Medical Devices (BfArM) to enter the self-testing list. After the COVID-19 Antigen Rapid Test (Colloidal Gold) for nose samples entered the BfArM self-testing list,it was once again approved by BfArM for the listing of self-testing market, becoming the only Chinese local IVD company on the list that has been approved for rapid testing of four self-testing antigens. The above products can be available in German supermarkets, pharmacies, Internet stores, etc. Special approval number: 5640-S-243/21 (special approval is valid until September 21, 2021).
Since the outbreak of the COVID-19, many countries have adopted PCR, rapid detection kits and other detection methods to develop and start to use vaccines and other methods to combat the epidemic. But at the same time, there are also more contagious virus variants, shortages of vaccines, shortages of medical institutions and other issues, which have caused the epidemic to repeat. Therefore, many European countries have begun to implement special approval channels for rapid detection and self-inspection of the COVID-19.
The German Minister of Health, Span, said that with the launch of self-testing kits, more people will have access to virus testing opportunities. If the virus infection can be detected as early as possible, the chain of infection can be cut faster and the virus can be prevented from spreading further.