



German Federal Health Minister Spencer said on February 16th that from March 1, Germany will be able to provide more rapid tests for the new coronavirus, and the public can receive the virus test again free of charge. At present, six new COVID-19 antigen (home-use) kits have been authorized for emergency use by BfArM. The certification means that the product can be sold in German supermarkets, and local residents can use the product for self-testing.
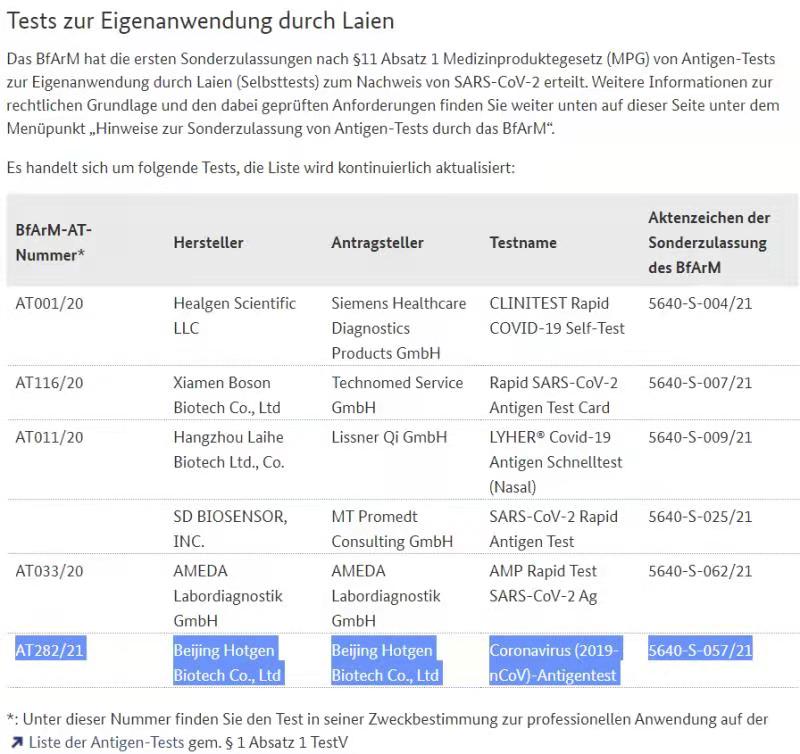
4 Chinese enterprise got the BfArM certificate and passed the evaluation of PEI are as follows respectively:
A. Healgen Scientific LLC affiliated to Zhejiang Orient Gene Biotech Co., Ltd.
B. Xiamen Boson Biotech Co., Ltd.
C. Hangzhou Laihe Biotech Ltd., Co.
D. Beijing Hotgen Biotech Co., Ltd.
The distributors in Germany of these 4 companies are as follows:
A. Orient Gene:
Siemens Healthcare GmbH
A. Menarini Diagnostics
TriPart Logistic GmbH
B. Hotgen Biotech
Dr. Grob Healthcare GmbH
C. Boson Biotech:
Technomed Service GmbH
MEDICE Arzneimittel P¨¹tter GmbH & Co. KG
L?we Medizintechnik
Medipro medizinische diagnostische Produkte GmbH
TREKSTOR GmbH
D. Laihe Biotech£º
Lissner Qi GmbH

Zhejiang Orient Gene Biotech Co., Ltd
Booth No. of 2021CACLP£ºN5-T035
Zhejiang Orient Gene Biotech Co., Ltd was established in December 2005. It is a company specializing in the R&D, production and sales of in vitro diagnostic products, belonging to the field of high-end medical equipment and devices. Currently, its main products are POCT instant diagnostic reagents, which are mainly used in the detection of infectious diseases (including COVID-19 detection products), drugs of abuse, fertility, tumor marker and cardiac marker detection, among which infectious disease and drugs of abuse detection are the two core product series of the company. The company¡¯s sales network has been extended to more than 120 countries and regions, and its overseas sales revenue accounted for 95% in 2019. At present, the company has obtained more than 300 overseas certifications/records, and more than 70 domestic registration/record certificates.

Beijing Hotgen Biotechn Co., Ltd.
Booth No. of 2021CACLP£ºN1-T005
Beijing Hotgen Biotechn Co., Ltd. (abbreviated as Hotgen Biotech, stock code: 688068) was established in June 2005, which is a high-tech enterprise focusing on the research& development, manufacture and sales of medical and public safety inspection products of in vitro diagnostics (IVD) in the field of biomedicine, as well as landed on the China Sci-Tech innovation board (STAR Market) in September 2019.
After serval years of Research& development, Hotgen Biotech has developed an in vitro diagnostic reagent bioactive raw material development platform, a sugar chain abnormal protein detection (sugar capture) R&D technology platform, a Magnetic particles chemiluminescence R&D technology platform, a Up-converting Phosphor R&D technology platform, and a colloidal gold immune layer, The eight major technology platforms, such as the precipitation R&D platform, enzyme-linked immunoassay R&D technology platform, molecular diagnostics R&D platform, and instrument R&D technology platform, form a closed-loop system for in vitro diagnostic R&D and production. Hotgen Biotech has established a complete full level immunodiagnostic technology platform, from high-precision Up-converting Phosphor POCT (UPT series) to small, medium and large single- cartridge chemiluminescence platforms (MQ60 series), and then to large-scale full-automatic chemiluminescence Platform (C2000), which realizes the application of the immune diagnostic platform in the field of full diagnostic scenarios. Supporting products are widely used in the clinical and public safety fields. Specific users include hospitals at all levels, township health centers, third-party testing centers, and medical institutions, as well as medical and health institutions, as well as disease control centers, public security, fire protection, military, ports, food and medicine. Supervision, food and feed enterprises and other public safety fields.

Xiamen Boson Biotech Co., Ltd.
Booth No. of 2021CACLP£ºN3-T051
Xiamen Boson Biotech Co., Ltd., as a specialist of in vitro diagnostic kits, develops and manufactures high-quality point of care and other immunoassay kits for the worldwide market. Our 12,500 square meter facility is operated strictly under ISO 13485:2016 and GMP guidelines. We combine the technological, material and labor resources of China and the US to develop, manufacture and supply high quality in vitro diagnostic products efficiently and cost effectively. Currently, we are offering product lines of rapid tests, CLIA, and raw materials. These product lines provide immunoassays in various formats to detect cardiac markers, drugs of abuse, fertility hormones, infectious diseases, tumor markers and animal diseases. Many of our products have been approved by the China NMPA.
Xiamen Boson Biotech¡¯s mission is to provide the affordable high-quality products to help fight diseases and illicit substance abuse.

Hangzhou Laihe Biotech Co.,Ltd.
Booth No. of 2021CACLP£ºN8-S014
Founded in 2012, Hangzhou Laihe Biotech Co.,Ltd.(hereinafter referred to as Laihe Biotech), a national high-tech enterprise, has always focused on the development and industrialization of POCT instant diagnosis, monitoring and health information technology field, and is committed to providing fast, accurate and reliable health detection products and services to public.
The company can continuously provide following products:
1. Drug Urine, Saliva, Hair Testing;
2. Inflammation (hs-CRP, PCT, CRP-SAA, PCR-IL6) Testing;
3. Cardiac Markers (cTn I, NT-proBNP, CMA) Testing;
4. Tumor Markers (AFP, CEA, PSA) Testing;
5. Torch Testing;
6. Novel Coronavirus (2019-nCoV) IgM/IgG Antibody Test and other Infectious Disease Testing.