



Original from: Abbott
Abbott is well known for its distinctive mix of growth and stability ¨C simultaneously innovating healthcare¡¯s next chapter and delivering strong growth as it returns value to shareholders ¨C and the fourth quarter was no exception.
Abbott finished 2024 with great momentum. Sales growth and earnings per share (EPS) growth in the fourth quarter were both the highest of the year. The company is harnessing that momentum as 2025 gets underway and is well-positioned to deliver another year of strong growth.
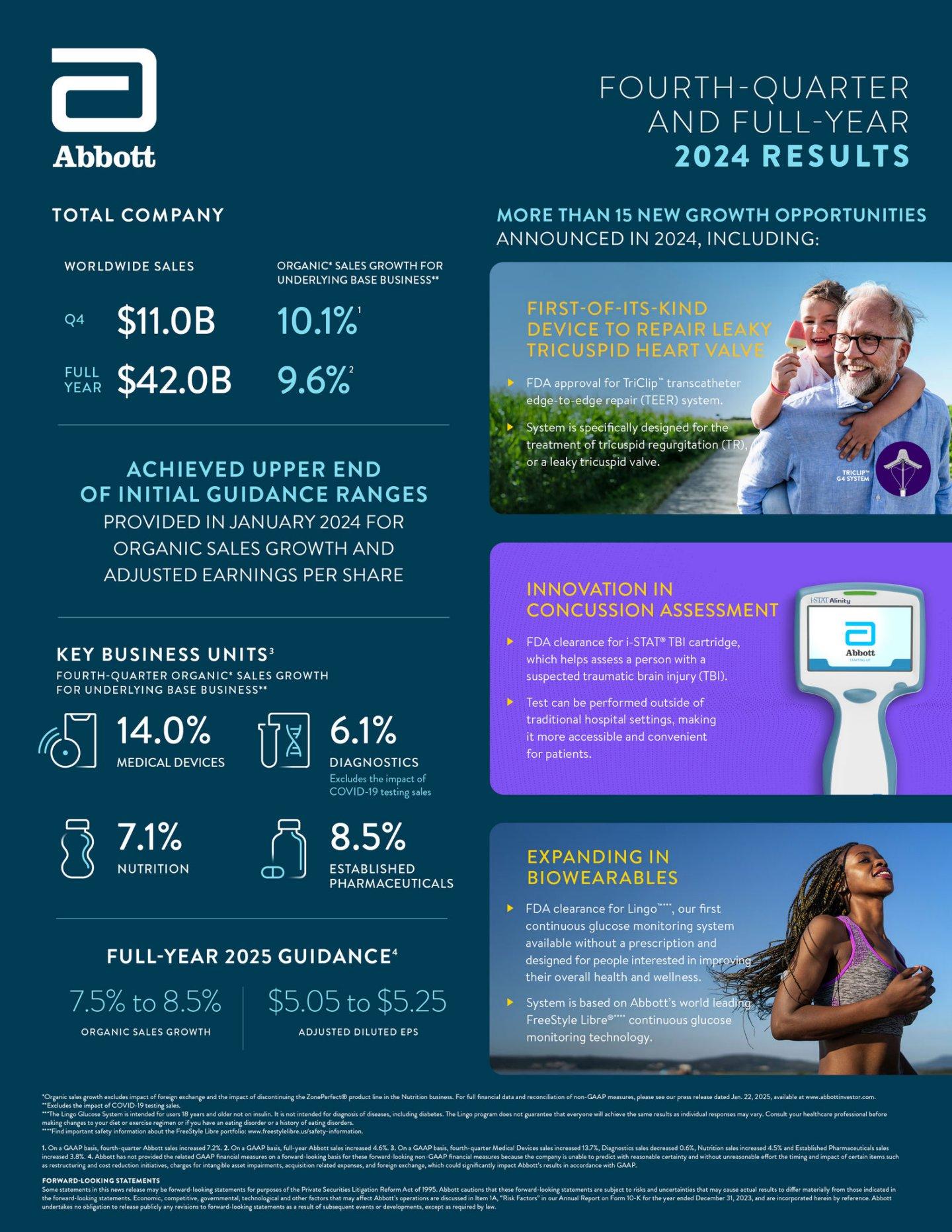
Full-year 2024 sales totaled $42.0 billion, a 9.6% increase on an organic basis1for the underlying base business2 (4.6% reported). Full-year adjusted diluted EPS was $4.67 (GAAP diluted EPS was $7.64).
Sales for the fourth quarter increased 10.1% on an organic basis for the underlying base business (7.2% reported), totaling $11.0 billion. The growth was led by a 14.0% organic increase (13.7% reported) in Medical Devices, where both Diabetes Care and Structural Heart revenue grew more than 20% in the fourth quarter.
Abbott¡¯s strong performance across all four business segments generated Q4 adjusted diluted EPS of $1.34 (GAAP diluted EPS was $5.27).
For the full-year 2024, Abbott achieved the upper end of the initial guidance ranges the company provided in January 2024 for both organic sales growth and adjusted EPS. The company also issued the following full-year guidance for 20253:
¡¤ Organic sales growth of 7.5% to 8.5%
¡¤ Non-GAAP operating margin of 23.5% to 24.0% of sales, which reflects an increase of 150 basis points at the midpoint
¡¤ Adjusted diluted EPS of $5.05 to $5.25, which reflects double-digit growth at the midpoint
Abbott¡¯s continued investments in R&D paid off in 2024, with more than 15 new growth announcements coming out of the highly productive pipeline. During the year, Abbott made advancements in care for people with heart conditions, suspected brain injuries and diabetes with the following FDA approvals:
¡¤ TriClip™ transcatheter edge-to-edge repair (TEER) system, a first-of-its-kind device to repair leaky tricuspid heart valve
¡¤ i-STAT® TBI cartridge, which helps assess a person with a suspected traumatic brain injury outside of traditional hospital settings
¡¤ Lingo™, our first continuous glucose monitoring system available without a prescription and designed for people interested in improving their overall health and wellness
Source: Q4 Momentum Positions Abbott for Strong Growth in 2025