



Original from: bioM¨¦rieux
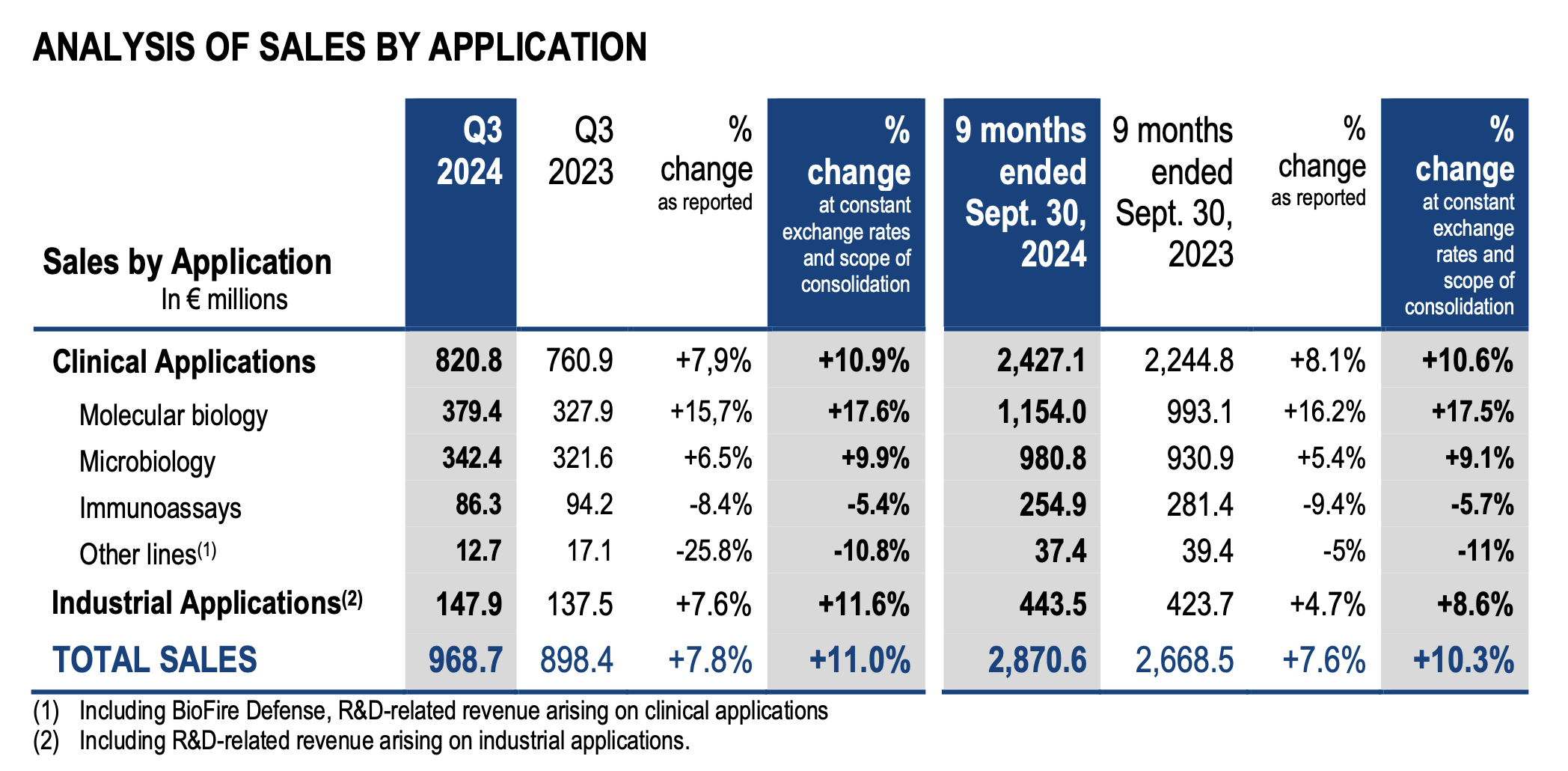
¡¤ +10.3% sales organic growth in the first nine months of the year with sales reaching €2,871m. A strong momentum fueled by the four growth drivers of the GO•28 strategic plan, growing +12%, together with a strong contribution from BIOFIRE®1 respiratory panels:
o BIOFIRE® non-respiratory panels sales are up +18% with all panels contributing strongly;
o Continued expansion of the SPOTFIRE®2 solution, reaching sales of €53m and a total installed base close to 2,100 instruments (+600 in Q3);
o Solid dynamic in microbiology, with sales increase of +9%, driven by a 13% growth in reagent sales thanks to volume uptake and prices increases;
o Industrial Applications sales are up +9%, with double digit growth in reagents sales in both food and pharma segments;
o BIOFIRE® respiratory panels sales increased +14%, illustrating the competitiveness
and the high medical value of the solution, and benefiting from a sustained respiratory epidemiology.
¡¤ +11% organic sales growth in Q3 2024 at €969m.
¡¤ Confirmation of the 2024 full year revised guidance with a slight update on the expected impact of the currency effect:
▪ Organic sales growth between +8% and +10% at constant exchange rates;
▪ Contributive operating income before non-recurring items (CEBIT)3 is expected to grow between +12% and +17% at constant exchange rate ;
▪ The currency effect3 is expected to have a negative impact in the range of around - €60m on the 2024 annual CEBIT versus around -€70m previously.
Pierre Boulud, Chief Executive Officer, said: ¡°bioM¨¦rieux delivered a dynamic performance in Q3 2024 in line with the strong results of the first half. Thanks to the very strong engagement from the bioM¨¦rieux team members, we continue to see positive momentum toward our GO•28 strategic plan targets. The double-digit growth in microbiology, non-respiratory BIOFIRE® panels and industrial applications quarterly sales, together with the deployment of our point-of-care solution SPOTFIRE®, demonstrate that we have identified the right drivers to pursue the development of bioM¨¦rieux. This also shows our portfolio meets the current public health needs. These positive achievements make us very confident in achieving our 2024 full year targets, that were recently revised upward.¡±
bioM¨¦rieux, a world leader in the field of in vitro diagnostics, today releases its business review for the nine months ended September 30, 2024.
SALES
Unless otherwise stated, sales growth is expressed at constant exchange rates and scope of consolidation (like-for-like).
Consolidated sales amounted to €2,871 million for the first nine months of 2024 versus €2,668 million for the prior-year period, representing a growth of +7.6% as reported. Organic growth (at constant exchange rates and scope of consolidation) reached +10.3% for the first nine months of the year. Changes in exchange rates had a negative €76 million impact on sales over 9 months, due to the appreciation of the euro especially against the Argentinian peso, the Turkish lira, Asian currencies and the American dollar.


EVENTS OF THIRD-QUARTER 2024
The USP Microbiology Expert Committee approves endotoxin testing using non-animal derived reagents
On July 26th, 2024, the Microbiology Expert Committee of the USP (US Pharmacopeia) has approved the inclusion of Chapter <86> Bacterial Endotoxins Test Using Recombinant Reagents, which permits the use of non-animal-derived reagents for endotoxin testing. Endotoxin testing is a critical step in ensuring the quality and safety of many sterile pharmaceutical products.
Based on recombinant Factor C (rFC), bioM¨¦rieux ENDONEXT™ technology eliminates the need to harvest horseshoe crab blood and provides reliable results everywhere from in-process controls to final product testing on the most complex matrices.
Internal verifications in the US
After 30 June 2024, in the framework of the Group¡¯s internal procedures, some internal control and compliance shortcomings have been identified within the Group's US operations. The Group has run additional verifications resulting in non-material financial impacts. These impacts have been integrated in the reported half-year statements. The Group continues to pursue its internal investigations and in parallel has started working on the implementation of actions to reinforce its internal control in the United States.
M-Pox: bioM¨¦rieux provides a real-time PCR detection kit called MONKEYPOX R-GENE®.
On August 14th, 2024, the World Health Organization (WHO) declared Mpox, a Public Health Emergency of International Concern which implies the highest level of epidemiological monitoring. To respond to this public health emergency, bioM¨¦rieux proposes a MONKEYPOX R-GENE® PCR kit. Available for Research Use Only (RUO), this kit can easily be used by laboratories around the world.
SUBSEQUENT EVENTS
bioM¨¦rieux receives CE-marking for VIDAS® VITAMIN B12 TOTAL, a blood test to measure total Vitamin B12 concentration
On October 15th, 2024, bioM¨¦rieux announced the CE-marking of VIDAS® VITAMIN B12 TOTAL, an automated quantitative test for use on the VIDAS® immunoassay instruments, for the measurement of total Vitamin B12 concentration in human serum or plasma. Vitamin B12, also known as cobalamin, is essential for well-being and cellular metabolism. This micronutrient, mainly present in food products from animal origin ¨C particularly meat, fish, eggs and dairy ¨C is required for normal functioning of the central nervous system, DNA synthesis and healthy red blood cell formation.
The commercial launch of VIDAS® VITAMIN B12 TOTAL is planned in selected countries at the end of 2024, with an extended rollout in Q1 2025.
bioM¨¦rieux and Oxford Nanopore Technologies signed an exclusive worldwide distribution agreement, for the AmPORE TB®, Research Use Only (RUO) assay, a molecular sequencing solution delivering a fast answer in the treatment of Mycobacterium Tuberculosis.
The AmPORE TB® RUO test based on targeted nanopore sequencing can detect Mycobacterium tuberculosis complex (MTBC) genetic variants associated with antimicrobial resistance in DNA extracted from sputum samples. bioM¨¦rieux will leverage its worldwide presence to distribute the solution, initially through a few trusted partner sites with a strong expertise in the diagnosis of tuberculosis and, then starting mid 2025 with a worldwide launch as a Research Use Only product.