



Original from: Roche
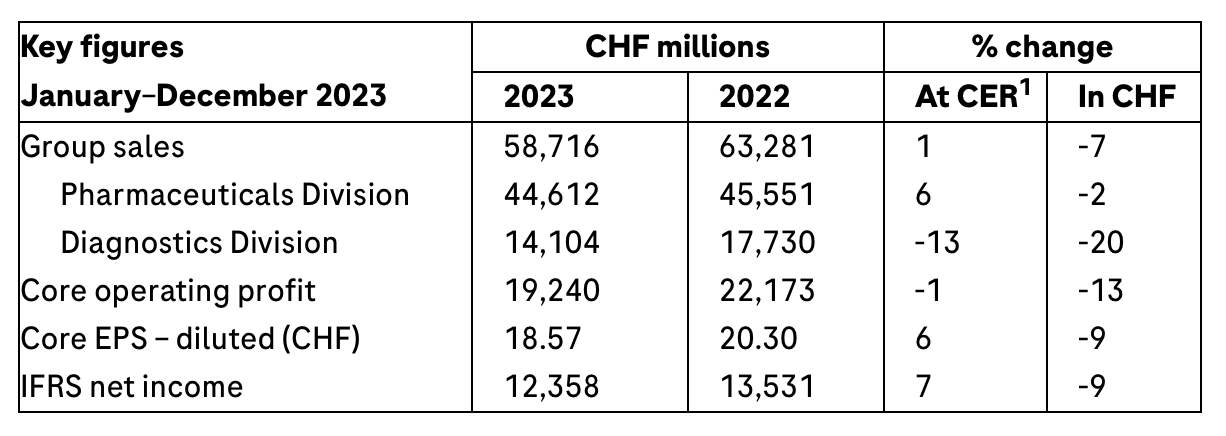
﹞ Group sales grow by 1%1 at constant exchange rates (CER; -7% in CHF), more than offsetting the decline in COVID-19-related sales and biosimilar erosion, and thereby exceeding 2023 guidance
﹞ Excluding COVID-19 products, Group sales increase by 8%
﹞ Pharmaceuticals Division sales increase by 6% (excluding COVID-19 medicine Ronapreve: +9%) due to ongoing high demand for newer medicines, with eye medicine Vabysmo continuing to be the top growth driver, followed by Ocrevus (multiple sclerosis), Hemlibra (haemophilia A) and Polivy (blood cancer)
﹞ Diagnostics Division sales are 13% lower due to high demand for COVID-19 tests in 2022; strong momentum in the Diagnostics Division*s base business continues with an increase of 7%
﹞ Highlights in the fourth quarter of 2023 and January 2024:
- US approval of Vabysmo (retinal vein occlusion, a severe eye disease)
- US priority review of Xolair (food allergies)
- EU approval of subcutaneous form of Tecentriq (cancer immunotherapy)
- Positive phase III data for inavolisib (breast cancer), Xolair (food allergies) and Hemlibra (babies with severe haemophilia A); positive longer-term data for Columvi and Lunsumio (blood cancer); positive long-term data for Kadcyla (breast cancer)
- US Breakthrough Device Designation for Elecsys NfL test (multiple sclerosis); launch of innovative new assays (hepatitis B and E)
- Acquisitions of Telavant (inflammatory bowel disease and other immunologic disorders) and Carmot (obesity and other metabolic diseases);
- acquisition agreement with LumiraDx (point-of-care technology platform)
- Roche (3rd) and Chugai (2nd) in Dow Jones Sustainability Indices
﹞ IFRS net income increases 7% (-9% in CHF) to CHF 12.4 billion
﹞ Core earnings per share increase by 6% (-9% in CHF)
﹞ Board proposes dividend increase to CHF 9.60
﹞ Change in Board of Directors
Roche CEO Thomas Schinecker: ※We achieved good sales growth that more than offset the sharp drop in COVID-19 sales. Roche*s base business 每 excluding COVID-19 每 continued its strong growth momentum with +8% at constant exchange rates. As a result, we exceeded our guidance for 2023. At the same time, the significant appreciation of the Swiss franc versus most currencies strongly impacted results when reported in Swiss francs. We also made good progress in both our pharma and diagnostics product pipeline. One recent highlight is inavolisib, an oral therapy investigated in phase III trials which showed a reduction of more than 50% in the risk of death or worsening disease for patients suffering from advanced, hard-to-treat breast cancer. We look forward to bringing this medicine to patients as soon as possible. Our new partnerships and acquisitions address disease areas with high unmet needs, such as inflammatory bowel disease and cardiometabolic disease. We are well positioned for future growth.§
Change in Board of Directors
Bernard Poussot (born 1952), who has been a member of the Board of Directors since 2015, has decided not to stand for re-election at the Annual General Meeting in 2024. All other members of the Board will be proposed for re-election.
Severin Schwan, Chairman of the Board: ※Bernard Poussot*s extensive leadership experience and profound knowledge of the pharmaceutical industry have resulted in significant contributions to Roche. I am sincerely thankful to him.§
Group results
In 2023, Roche achieved sales growth of 1% (-7% in CHF) to CHF 58.7 billion, exceeding the company*s guidance for the year. This increase more than made up for the expected decline in COVID-19-related sales, amounting to CHF 4.3 billion, and the effects of biosimilar erosion on our cancer medicines MabThera/Rituxan, Herceptin and Avastin, which totalled CHF 1.1 billion 每 resulting in an overall impact of CHF 5.4 billion (at CER).
The Swiss franc significantly appreciated versus most currencies, impacting results when reported in Swiss francs compared to constant exchange rates.
Core operating profit was down 1% (-13% in CHF) to CHF 19.2 billion. The good sales performance and a return towards a pre-COVID-19 sales mix resulted in an improved gross margin. This was offset by continued investments in pharmaceutical research, development and launches of new products. Additionally, the patent settlement income in Japan in 2022 affected the growth rate in 2023.
IFRS net income increased by 7% (-9% in CHF) to CHF 12.4 billion due to the increase in operating profit (IFRS) and lower income tax expenses.
Core earnings per share rose by 6% (-9% in CHF), including the positive impact from the resolution of tax disputes in 2023.
Sales in the Pharmaceuticals Division increased by 6% to CHF 44.6 billion, with newer medicines for severe diseases continuing their strong growth.
The top five growth drivers 每 Vabysmo (severe eye diseases), Ocrevus (multiple sclerosis), Hemlibra (haemophilia A), Polivy (blood cancer) and Phesgo (breast cancer) 每 achieved total sales of CHF 14.8 billion. This represents a plus of CHF 4.3 billion at CER compared to 2022.
Vabysmo, launched only in early 2022, reached sales of CHF 2.4 billion, and has become one of the best-selling medicines of Roche.
In the United States, sales rose by 8%. Vabysmo, Ocrevus and Hemlibra were the main growth drivers. This increase was partly offset by declining sales of medicines with expired patents.
Sales in Europe grew by 6%, with key contributions from Germany, France and the UK. The sales growth of Vabysmo, Phesgo, Evrysdi (spinal muscular atrophy), Hemlibra and Ocrevus more than compensated for the lower sales of Ronapreve (COVID-19) and medicines with expired patents.
In Japan, sales were down by 14%, mainly due to lower supply of Ronapreve to the government. Excluding Ronapreve, sales in Japan grew by 6%. This increase was fueled by the strong performance of newer medicines such as Polivy and Vabysmo, which effectively compensated for the impact of biosimilars.
Sales in the International region grew by 13%, led by China, Brazil and Canada. In China, sales rose by 6%, with Tamiflu (influenza), Xofluza (influenza) and the cancer medicines Polivy, Tecentriq and Perjeta being the key growth drivers, more than offsetting the impact of biosimilars and lower sales of CellCept (transplantation).
The Diagnostics Division*s base business sales increased by 7%, with immunodiagnostic products, particularly cardiac tests, and diagnostics solutions for clinical chemistry and for advanced staining contributing significantly to this growth.
Overall, the Diagnostics Division reported sales of CHF 14.1 billion, a decline of 13%. This reflects the anticipated significant drop in demand for COVID-19-related products (sales of CHF 0.8 billion in 2023, compared to sales of CHF 4.1 billion in 2022, both at CER).
With lower demand for COVID-19 testing, sales in the North America, EMEA and Asia-Pacific regions decreased by 21%, 13% and 11%, respectively. The division's base business grew across all regions.