



Original from: MDCLOUD
The total number of first-time registrations of Class II and III medical devices in 2023 was 14,942, a decrease of 129 registrations compared with that of 2022.
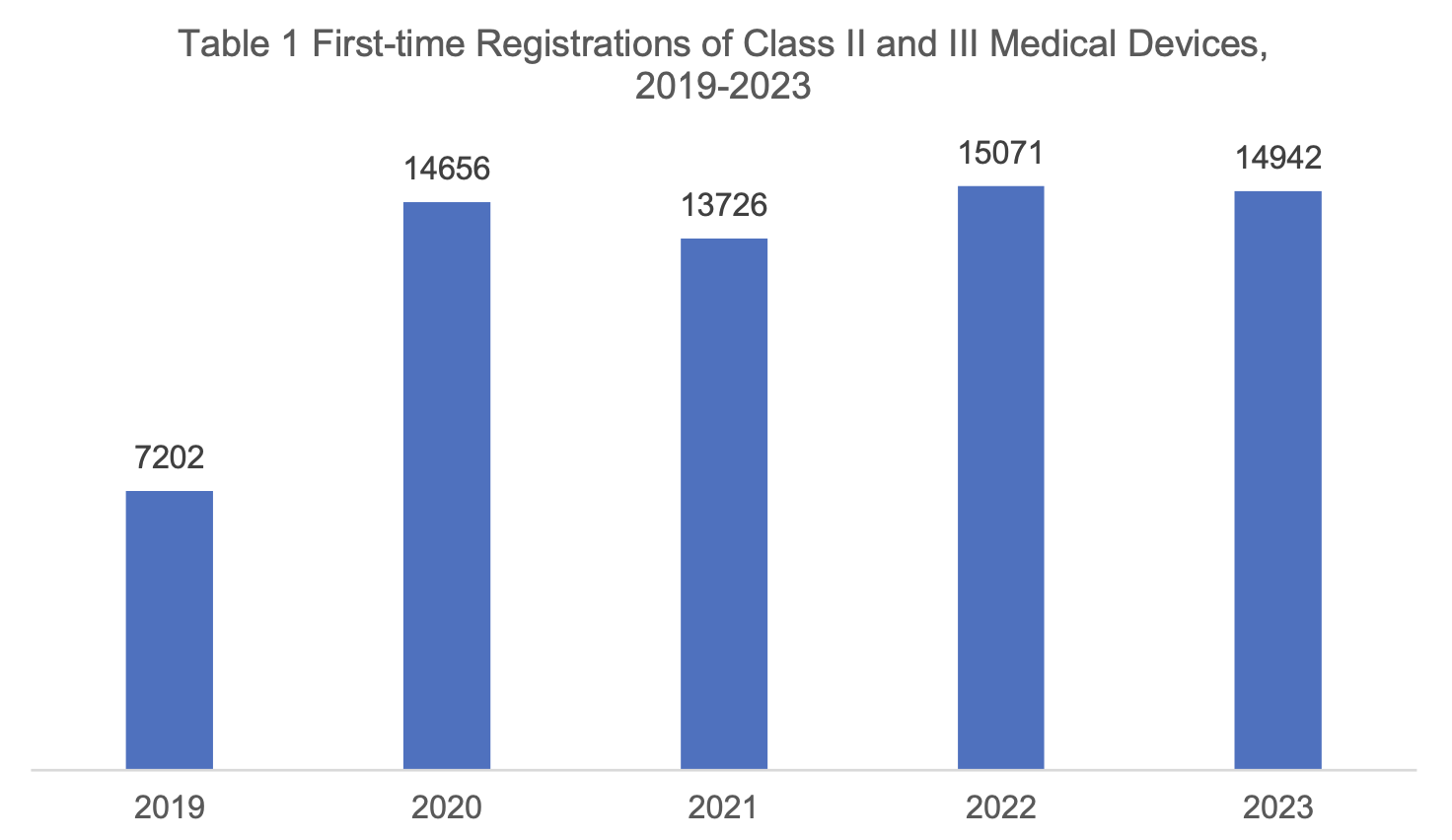
Among them, 12,935 pieces of Class II products were registered for the first time, and 2007 pieces of Class III products were registered for the first time.
1 Segmentation
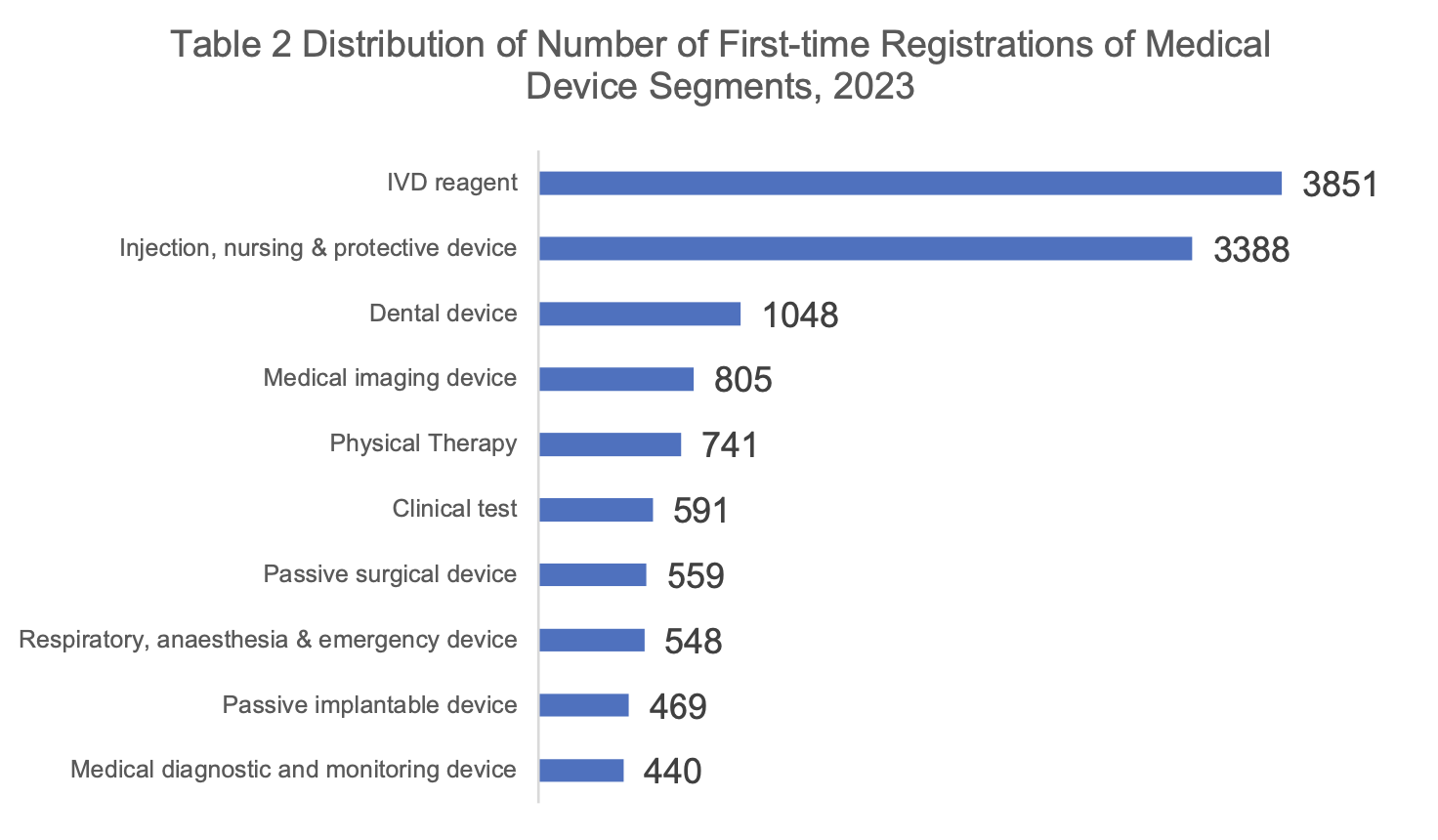
IVD reagents maintain the leading position in the number of first-time registrations.
In terms of segmentation, the first registrations of Class II and III products ranked first for IVD reagents, totaling 3,851, followed by infusion, nursing and protective devices (3,388) and stomatological devices (1,048).
2 Region
2.1 Province
Guangdong and Jiangsu lead in the number of first-time registrations.
In terms of the number of first-time registrations of Class II and III products in each province, Guangdong (2,488) and Jiangsu (2,257) ranked first and second in 2023.
Hunan and Henan ranked third and fourth with 1,282 and 1,024 respectively, followed by Beijing, Shandong, Zhejiang, Hubei, Chongqing and Jilin.
2.2 City
In terms of city ranking, Shenzhen ranked first with 1,285 first registrations of Class II and III medical devices, while Beijing and Changsha ranked second and third with 935 and 875 registrations respectively.
2.3 Growth Rate
In 2023, the first growth rate of the number of first registrations of medical device products by province is Tibet, with a growth rate of 400%, while Xinjiang and Shanxi are the second and the third with 200% and 136% respectively.
In 2023, the first growth rate of the number of first registrations of Class II medical devices is Tibet with a growth rate of 400%.
Chongqing is the number of first registrations of Class III products grew at a rate of 425%.
In 2023, the first growth rate of the number of first registrations of Class III medical devices is Gansu with a growth rate of 150%.