



1. Overall
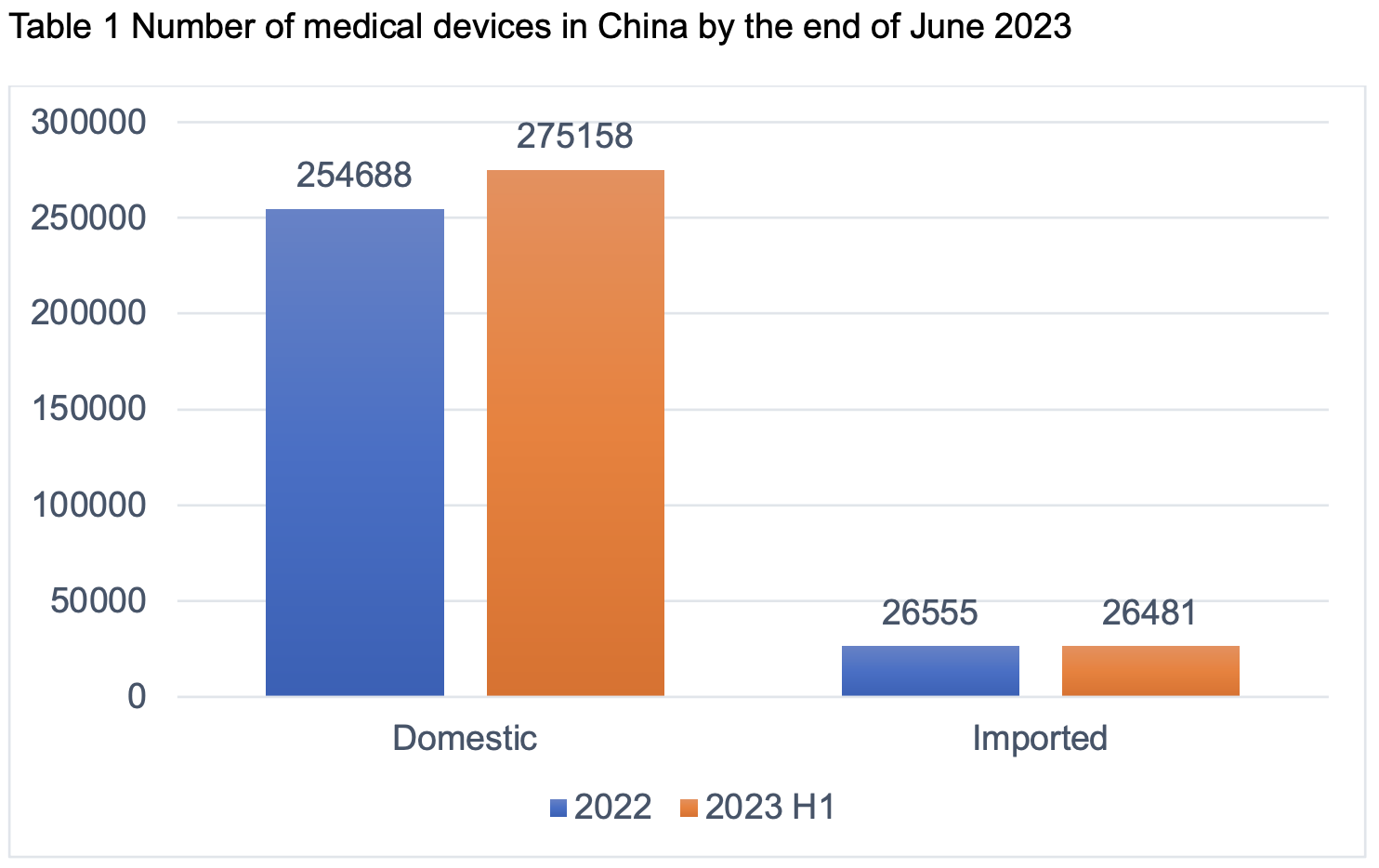
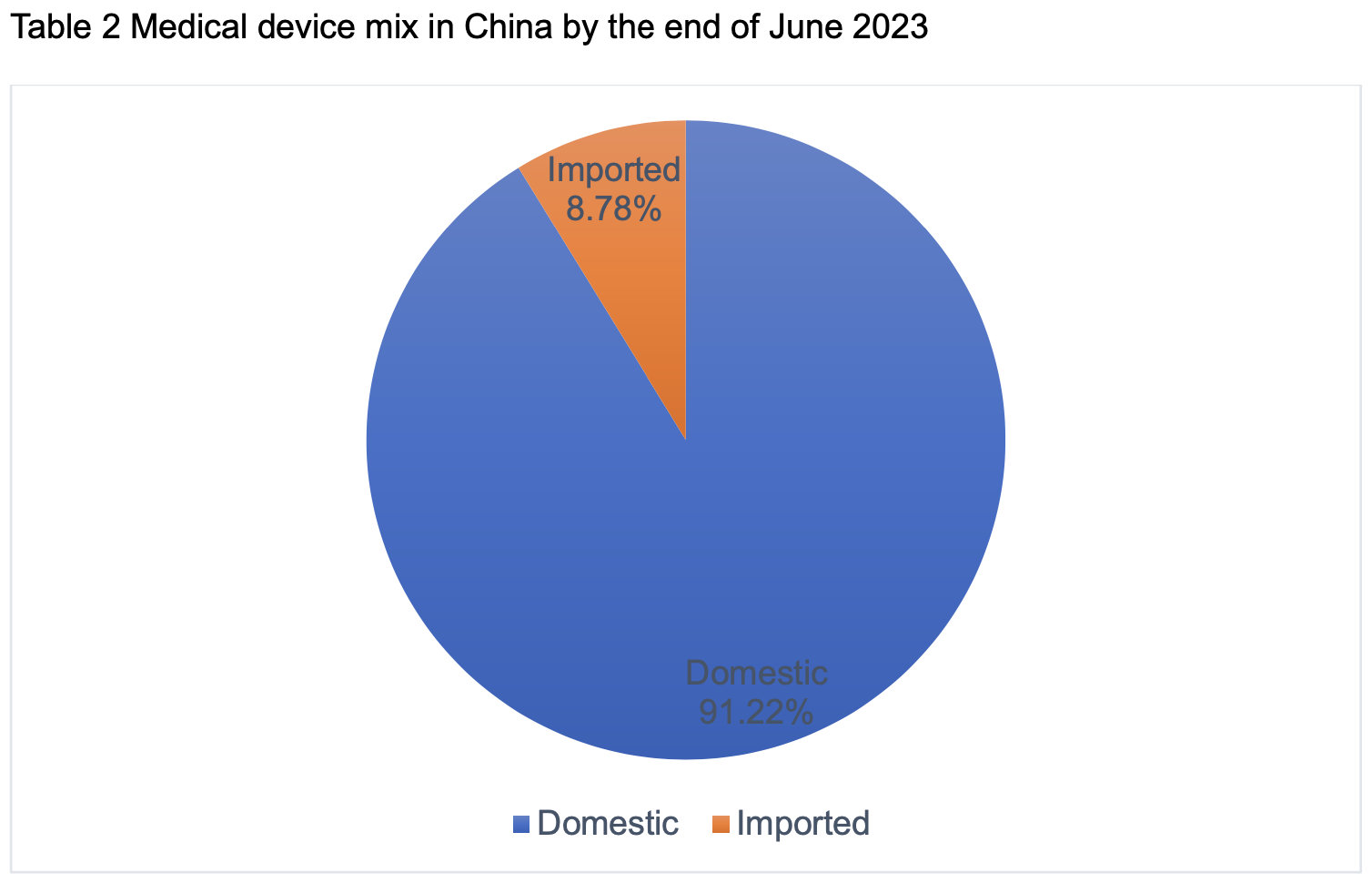
Based on the statistics from JOINCHAIN, as of the end of June 2023, the number of valid registrations and filings of medical devices nationwide reached 301,639, an increase of 18.12% compared with the same period of last year, with 46,283 new cases, an increase of 7.25% compared with the end of 2022 (281,243 cases). Among them, there are 275,158 domestic products and 26,481 imported products, with imported products accounting for 8.78%.
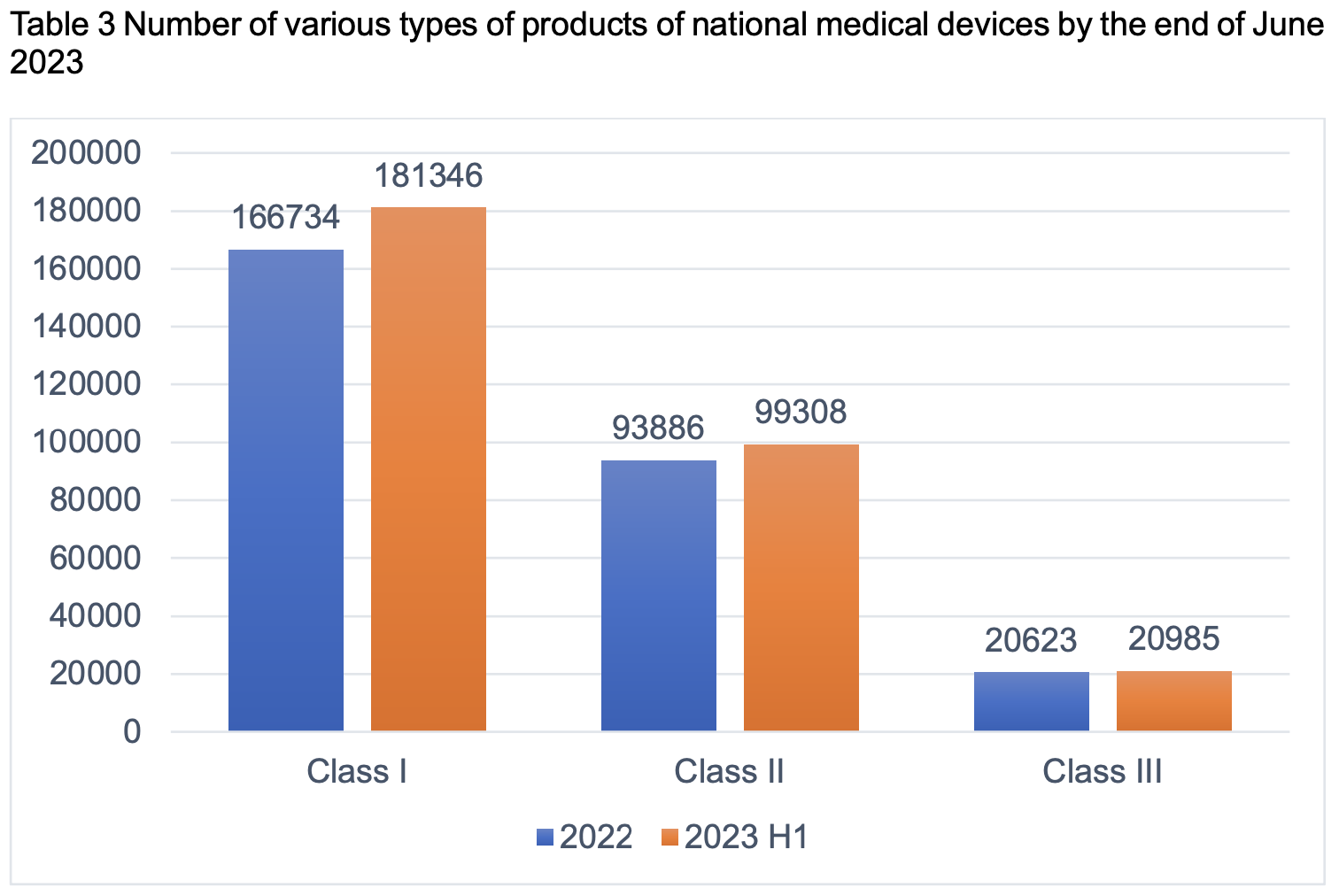
From the perspective of product category, there are 181,346 pieces of Class I products, accounting for 60.12%; 99,308 pieces of Class II products, accounting for 32.92%; and 20,985 pieces of Class III products, accounting for 6.96%.
2. New products
2.1 Product registration & filing
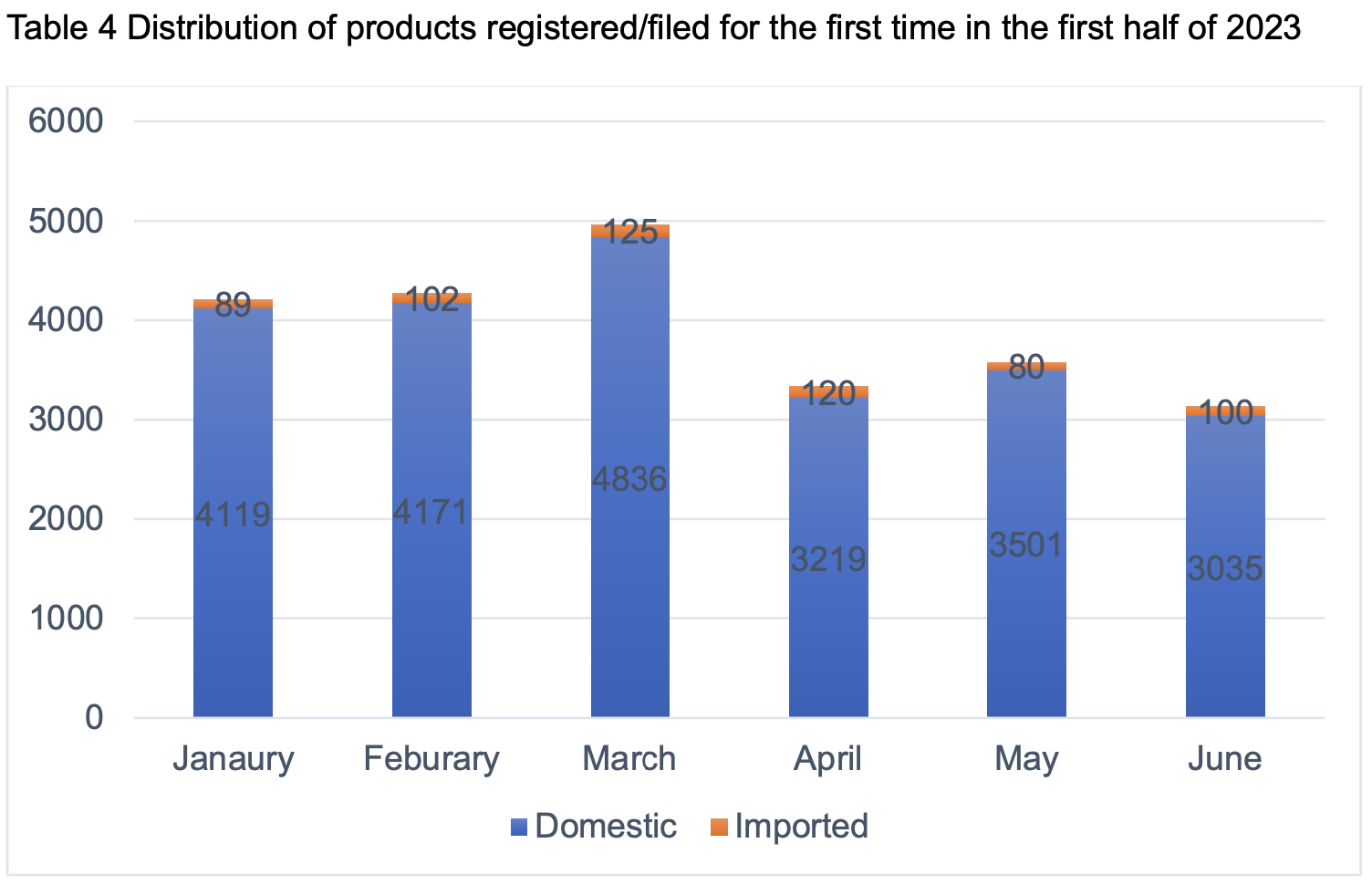
In the first half of 2023, the number of products registered/filed for the first time reached 23,497, of which 22,881 were domestic products and 616 were imported products.
2.2 Segmentation
In the first half of 2023, the top three medical device types in terms of the number of first registered/filed products were in vitro diagnostic reagents (9,039), injection, care and protection devices (3,742), and stomatological devices (1,479).
3. Import Registration Procedures
As one of the most promising medical device market and one of the largest markets in the world, China becomes the targeted market of many international medical device companies and they hope to obtain the access to the Chinese market in accordance with the regulatory requirements of medical device registration.
3.1 Regulatory basis for registration of imported medical devices
There are two main regulatory basis for the registration of imported medical devices: Medical Device Supervision and Administration Regulations and Medical Device Registration Management Measures.
On the basis of these two regulations, China has introduced a system of administrative regulations, standards used to regulate the import and guide the registration of medical devices.
3.2 Imported medical devices registration flow chart
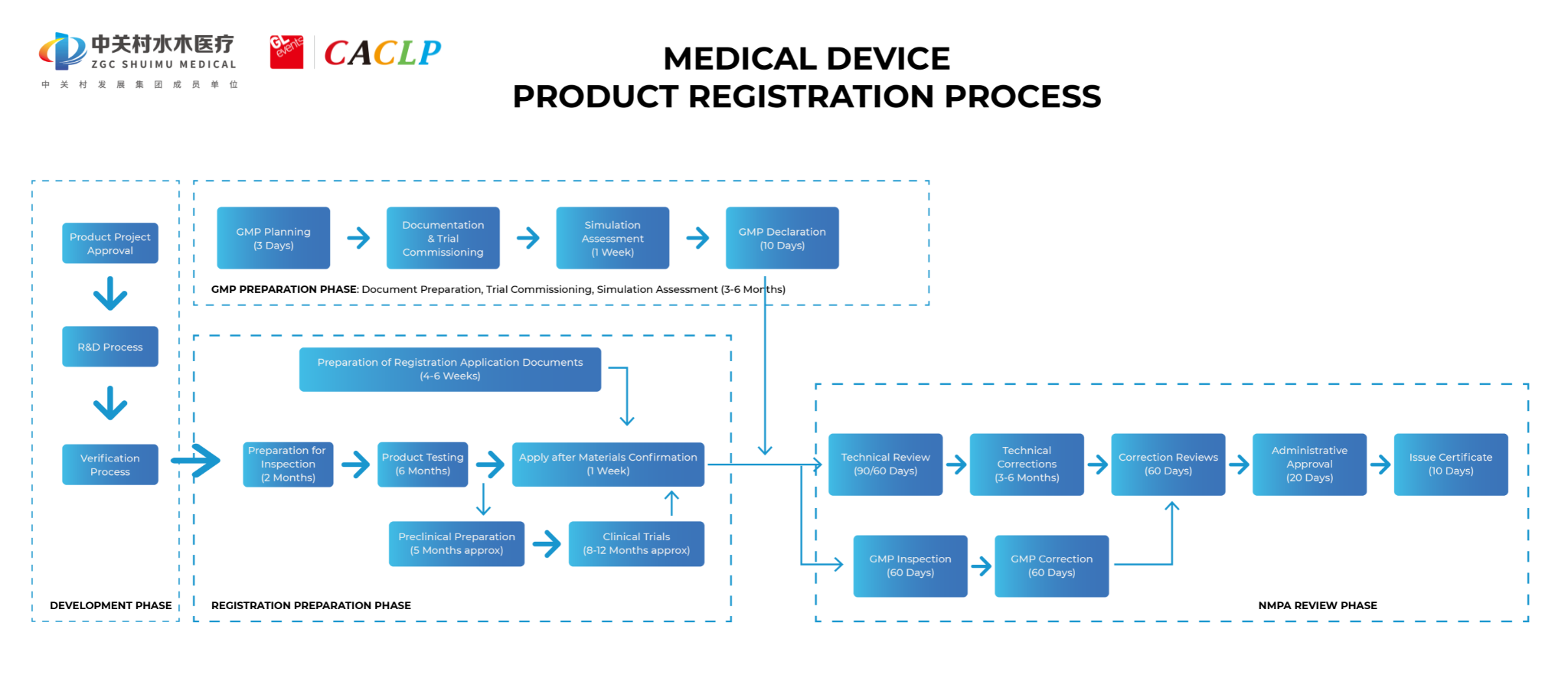
3.3 Catalogue of materials to be submitted by the applicant for registration of imported medical devices
File 1. Overseas medical device registration application form
File 2. Medical device manufacturer qualification certificate
File 3. A copy of the business license of the applicant and the power of attorney granted by the manufacturer to act as an agent for registration
File 4. Documents of overseas government medical device authorities approved or recognized the product as a medical device to enter the country (region)
File 5. The applicable product standards
File 6. Medical device instructions
File 7. The product registration test report issued by medical device testing organizations (applicable to the Class II and III medical devices)
File 8. Medical device clinical trial information
File 9. The production of product quality assurance issued by the enterprise
File 10. The manufacturer in China to designate the agent's power of attorney, the agent's letter of commitment and business license or certificate of registration
File 11. Power of attorney appointing the after-sales service organization in China, letter of commitment from the entrusted organization and qualification documents
File 12. Self-assurance statement of the authenticity of the submitted materials