As the demand for the mass spectrometry market in China has rapidly increased, major domestic and international mass spectrometer manufacturers have accelerated their product registration efforts. In recent years, a variety of different types of mass spectrometry instruments have obtained NMPA certificates.
The National Medical Products Administration initially approved the clinical application of mass spectrometry technology in 2008. In the early days, mass spectrometry technology was primarily focused on newborn genetic metabolic disease screening using tandem mass spectrometry. Starting in 2017, with the efforts of various industry players including mass spectrometry manufacturers, reagent manufacturers, and third-party independent laboratories and institutions, the application of mass spectrometry in clinical diagnostics has become increasingly widespread and deep-rooted. With the rapid increase in awareness and acceptance of mass spectrometry technology in major domestic hospitals, the clinical mass spectrometry industry experienced explosive growth from 2019 to 2023.
From 2019 to September 14, 2023, a total of 92 mass spectrometry instruments have obtained certification. Among them, 14 certificated instruments come from seven overseas companies. The growth rate in 2021 and 2022 was particularly fast, with the number of registrations increasing by more than 50% compared to the previous year.
The main types of mass spectrometry used in clinical diagnostics include LC-MS/MS, MALDI-TOF-MS, ICP-MS, LC-QTOF, and GC-MS. Among these, LC-MS/MS is the most widely applied mass spectrometry technology in clinical diagnostics. Its applications primarily focus on four major areas: vitamins, therapeutic drug monitoring (TDM), endocrinology, and newborn disease screening, accounting for over 90% of the clinical mass spectrometry market for LC-MS/MS.
LC-MS/MS uses liquid chromatography as the separation system and mass spectrometry as the detection system. Samples are separated in the mobile phase and mass spectrometry section, ionized by mass spectrometry, and the ion fragments are separated by mass-to-charge ratio in the mass analyzer. This process results in a mass spectrum.
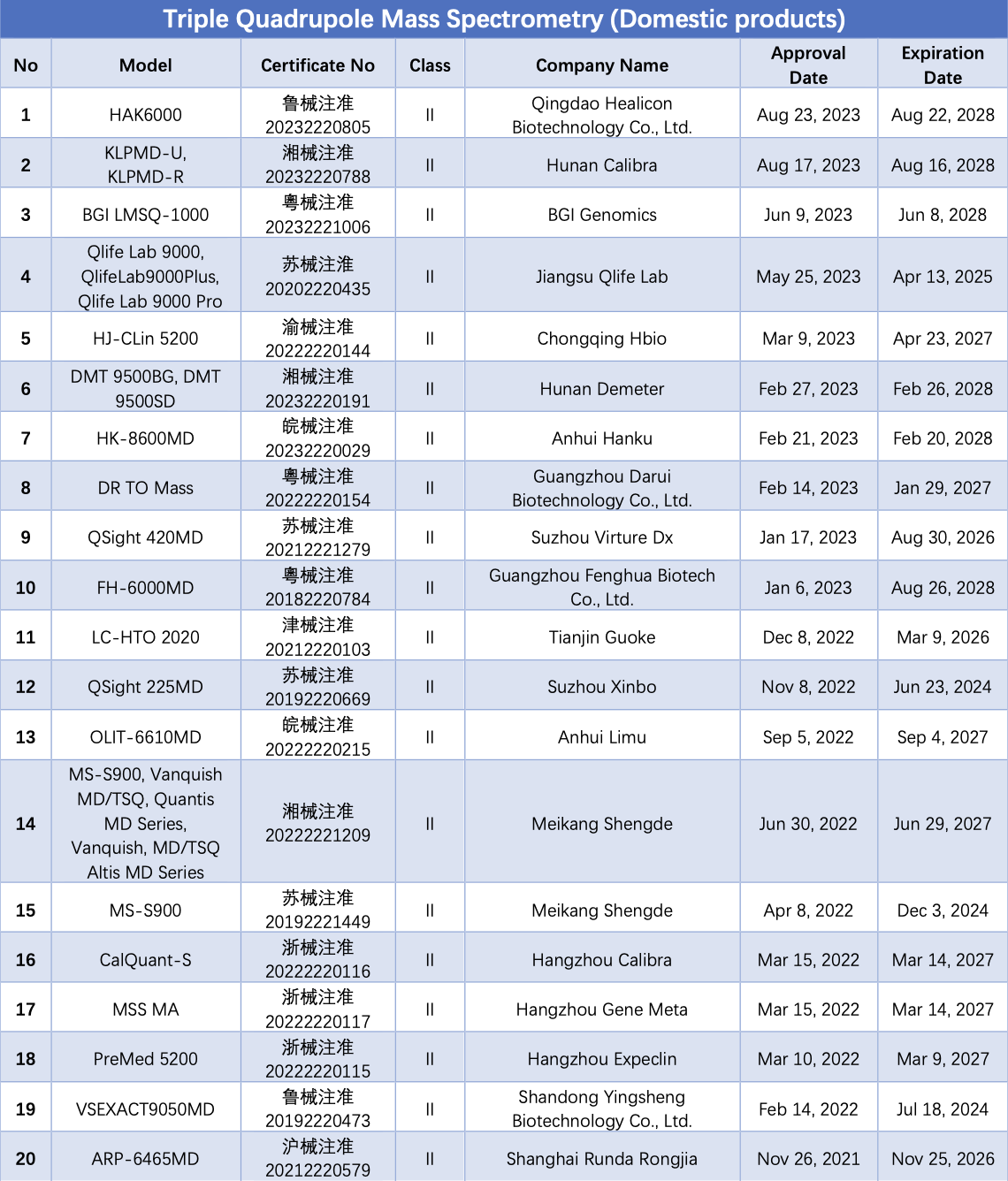
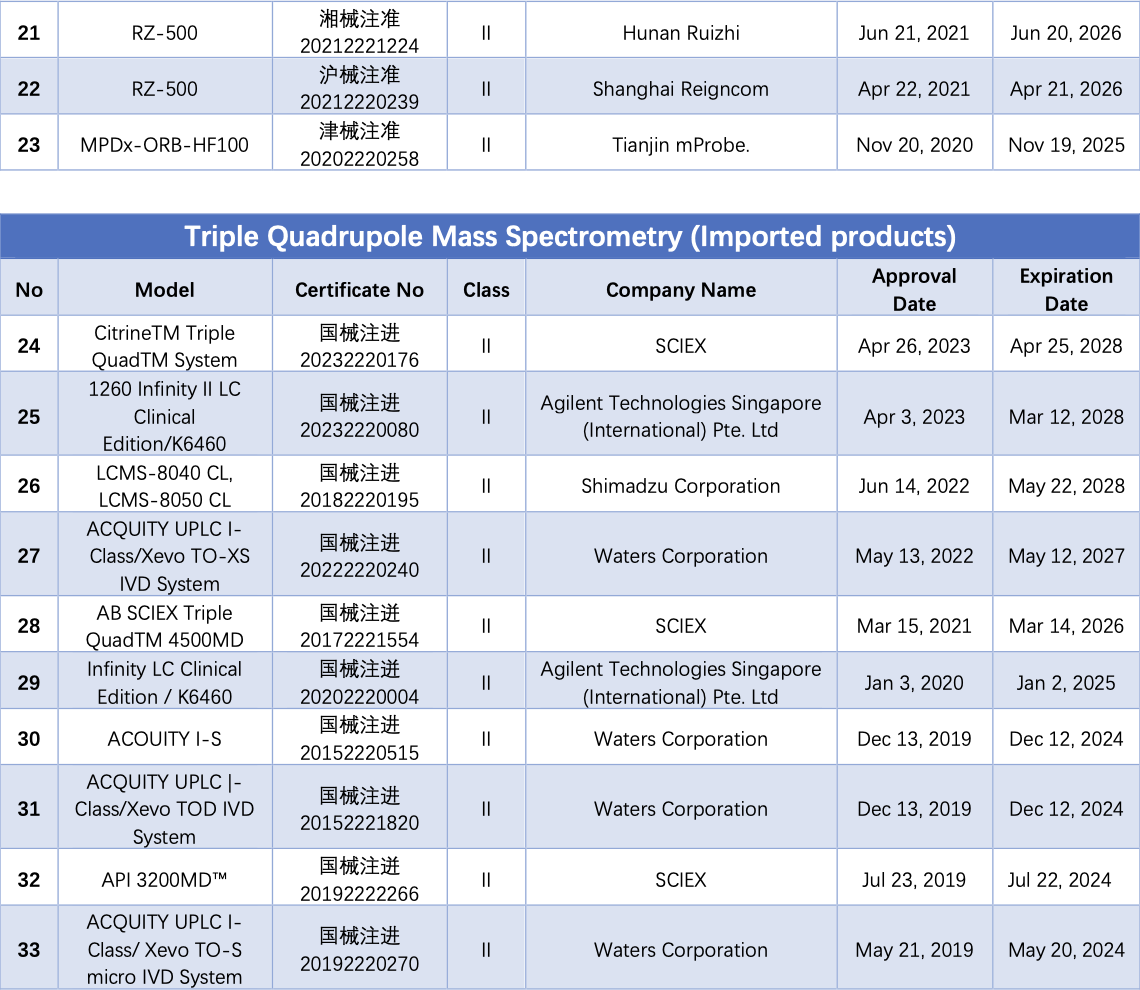
As of September 14, a total of 40 LC-MS/MS instruments have obtained NMPA registration certificates. Among them, 29 are domestically produced (including OEM), and 11 are imports. Analysis of the structure of liquid chromatography tandem mass spectrometry detection systems that have received NMPA certification reveals that many of them employ triple quadrupole mass spectrometry and high-performance liquid chromatography in tandem.
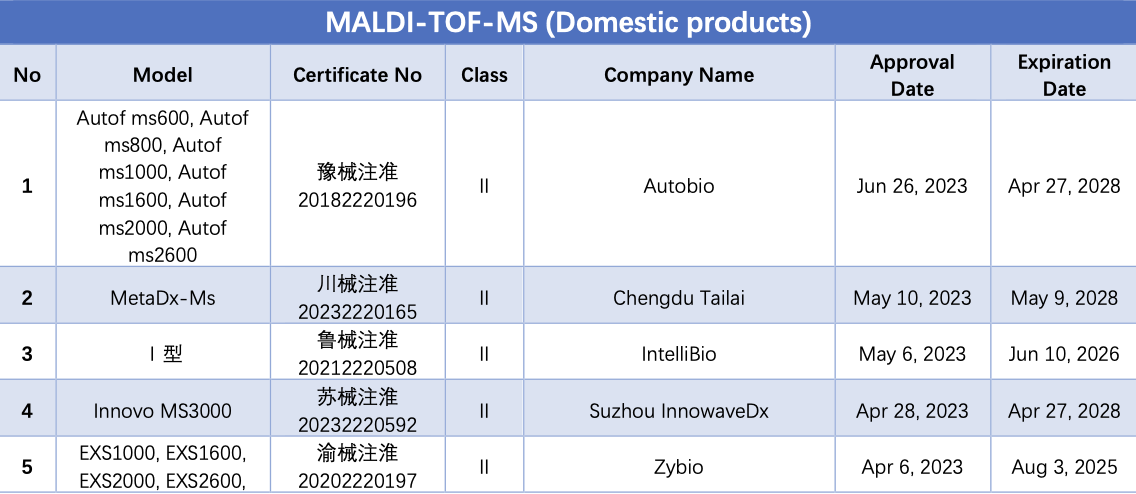
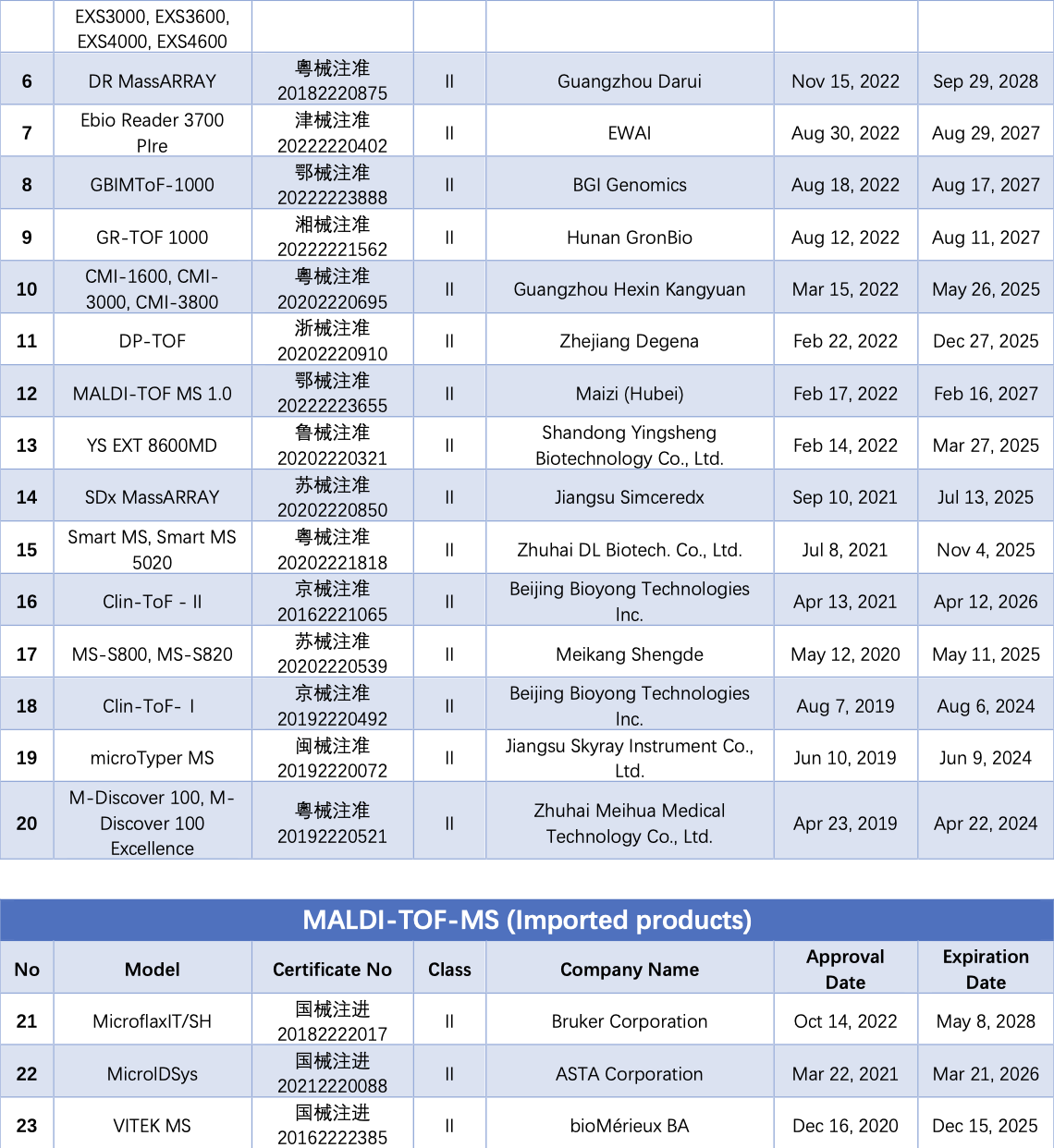
MALDI-TOF-MS is one of the most representative technologies in clinical microbial identification, holding significant potential for detecting large molecular biomarkers such as proteins, nucleic acids, and lipids. Each microorganism has a unique peptide/protein composition, and by detecting the peptide/protein fingerprint using MALDI-TOF-MS, and comparing it with a microbial database through software processing, identification at the species and genus levels can be completed in minutes.
As of September 14, a total of 38 MALDI-TOF-MS instruments from over 20 companies have obtained NMPA certificates. Among these, 35 are domestically produced, and 3 are imports.
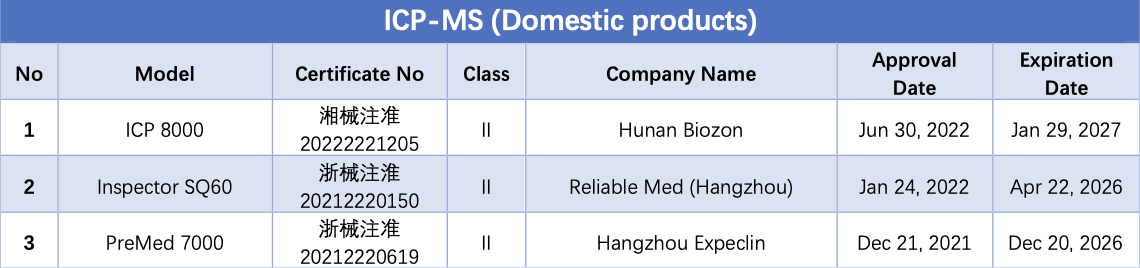
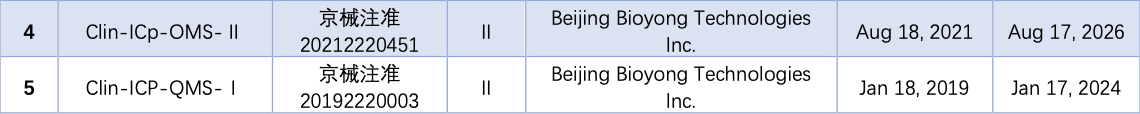
ICP-MS (Inductively Coupled Plasma Mass Spectrometry) is an inorganic mass spectrometry technology that simultaneously detects trace amounts of multiple elements while using inductively coupled plasma as the ion source. ICP-MS boasts high sensitivity, a wide linear range, and the ability to simultaneously analyze multiple elements, making it the gold standard in trace element analysis.
As of September 14, a total of 5 ICP-MS instruments have obtained NMPA certificates, all of which are domestically produced.
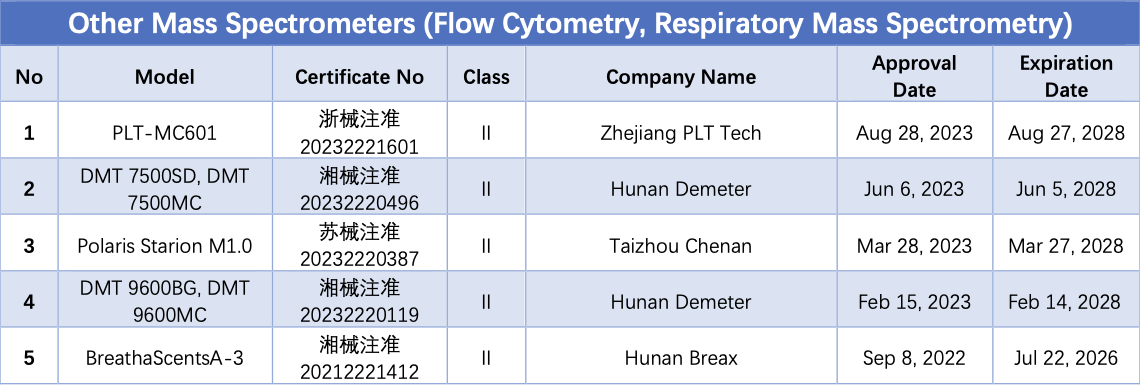
In addition to the above common types of mass spectrometers, as interdisciplinary technologies continue to develop and application areas expand, mass spectrometry technology, when combined with other advanced analytical and detection technologies, has gradually given rise to new detection techniques and application directions. These include mass spectrometry flow cytometers, two-dimensional liquid chromatography mass spectrometry, mass spectrometry imaging, and human breath gas detection mass spectrometry.
Currently, there are 2 flow cytometry mass spectrometers, 2 miniature mass spectrometers, and 1 human breath gas detection mass spectrometer that have obtained NMPA registration certificates.