



Original from: IVD News
Digital PCR (dPCR) is the third-generation PCR technology, which is key to life science and medical diagnosis.
The core idea is to dilute the template molecules and evenly distribute them into tens or hundreds of thousands of independent reaction units for PCR amplification, and to achieve absolute quantification of single molecules independent of standards and standard curves through the PCR endpoint signal "yes or no".
Compared with the second-generation fluorescent PCR technology, dPCR has advantages in high sensitivity, accurate quantification, and high stability.
Digital PCR Market
According to the forecast of GHICapital, the market size of China's dPCR industry will still maintain a high growth rate from 2020 to 2024, and the market size will grow from 2.133 billion yuan to 7.011 billion yuan, at a CAGR of 34.65%.
Internationally, companies such as Bio-rad, Qiagen, Thermo Fisher, Roche, Illumina have laid out their dPCR business through acquisitions and investments.
In China, IVD companies such as TargetingOne Corporation, RainSure Scientific, Pilot Gene, Turtle, Zhenzhun Bio, Kwins, Forevergen, Sniper have been born, and listed IVD companies such as Maccura, Snibe, and ABP have all invested in dPCR companies.
Application
1. Early and rapid diagnosis of infectious diseases. The use of dPCR with high sensitivity and absolute quantification enables dynamic monitoring of pathogenic microorganisms and drug-resistant genes, providing a reference for the development and adjustment of clinical protocols.
2. Tumor genetic testing provides reliable testing products for early warning, prognosis, efficacy prediction and evaluation of lung cancer, breast cancer, gastrointestinal tract tumors, thyroid cancer, and other diseases.
With its advantages of ultra-high sensitivity and absolute quantification, digital PCR is particularly suitable for liquid biopsy of tumors and can overcome the shortcomings of low content of tumor-derived nucleic acids and complex background in such specimens and has good application prospects in the dynamic monitoring of minute residual disease (MRD), efficacy evaluation, disease progression and clonal evolution.
3. Birth defects screening, which allows faster, cheaper, and more accurate screening for chromosomal disorders such as Down's syndrome and genetic disorders such as SMA (Spinal Muscular Atrophy).
Digital PCR technology, which ensures the accuracy of chromosome copy number quantification with relatively rare sample sizes, meets the technical needs of Down syndrome screening. For SMN2 gene copy number detection is accurate, rapid, and economical, which can meet the needs of SMA clinical genetic testing and prenatal diagnosis.
The advanced and important value of digital PCR technology has become an industry consensus.
With the continuous growth of the testing market and increasing testing requirements, dPCR is bound to become a key technology platform for clinical molecular diagnostics and usher in rapid growth.
China¡¯s national public bidding results show that since 2013, the number of digital PCR annual tender from single digit into the annual tender volume of more than 100, especially in 2021 and 2022, the number of digital PCR reached more than 300 sets of winning in each year.
From the universities and research institutes, customs, CDC, hospitals, and other major fields are in the layout of the digital PCR. Besides, the industrial sector, including molecular diagnostics, metrology and biopharmaceuticals, is more favorable to digital PCR technology. Therefore, digital PCR has become a technology recognized and affirmed by the state and the whole industry, with huge space for future development.
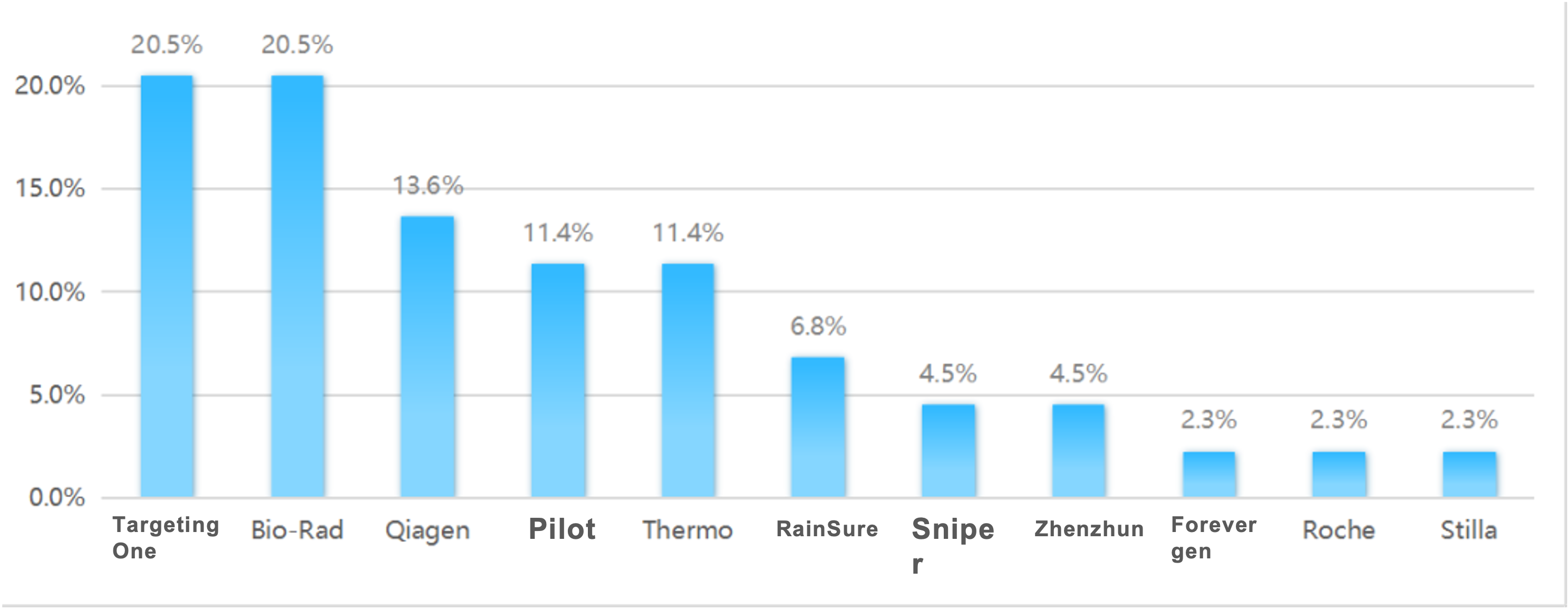
2022 Digital PCR Instrument Winning Bid Data
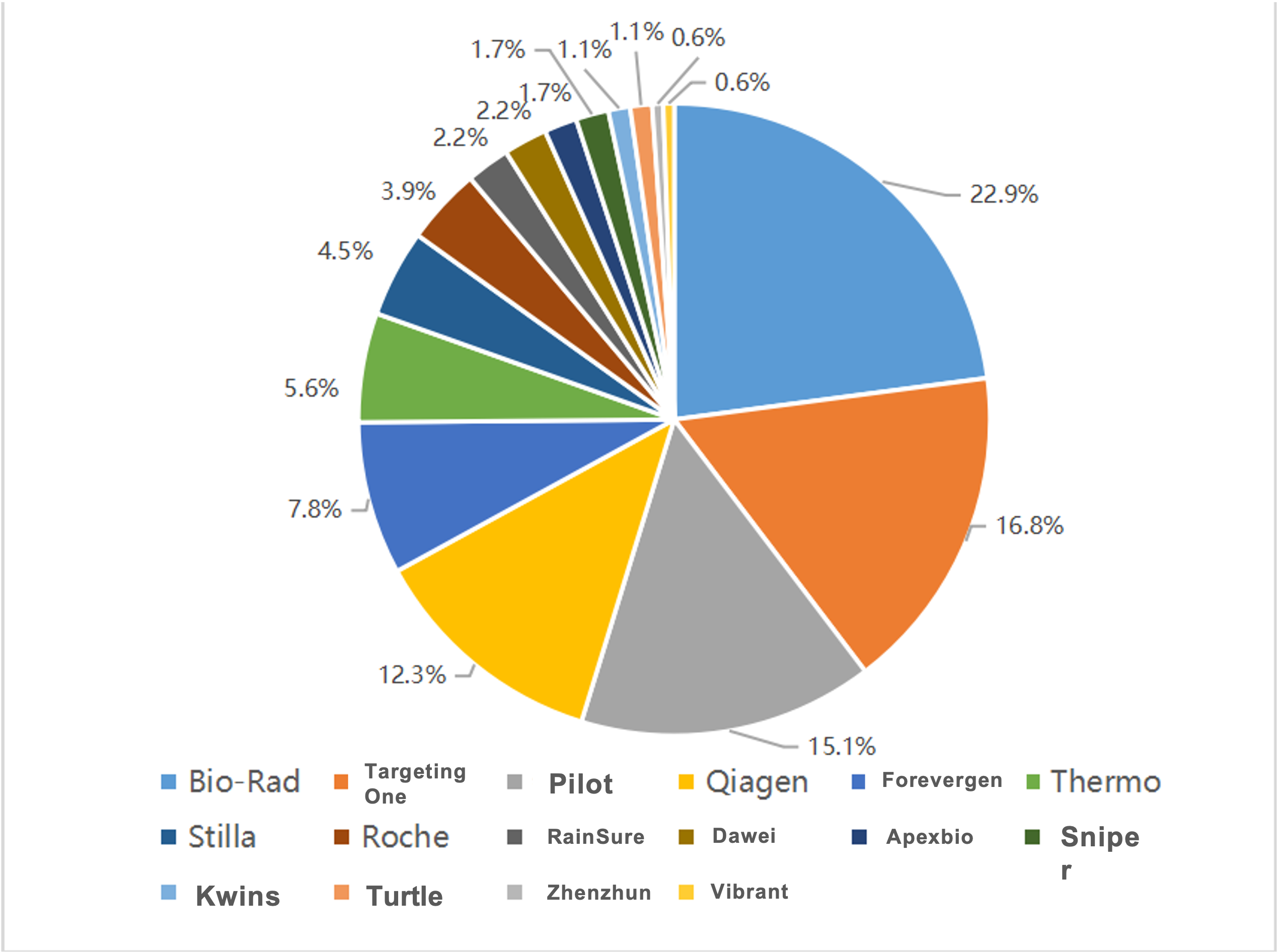
2023 Jan-Jun Digital PCR Instrument Winning Bid Data
Policy
Recently, the National Development and Reform Commission issued the "Announcement on the Public Consultation on the <Guidance Catalogue for Industrial Structure Adjustment (2023 Edition, Draft for Comments)> that mentioned about new gene, protein and cell diagnostic equipment, new medical diagnostic equipment and reagents.
The "14th Five-Year Plan" for the development of medical equipment industry focuses on the development of diagnostic equipment as well.
In terms of advancement and innovation, digital PCR is the key innovation direction encouraged by the state.
Recognitions from the state and the industry, accelerated reagent certification and the layout of digital PCR by worldwide brands and listed companies have strengthened the industry's confidence in digital PCR. Digital PCR is very likely to lead the development of molecular diagnostics in the next 10 years.