



China has been reopened. The world¡¯s second largest economy, which plays an important role in accelerating domestic economic recovery as well as boosting global growth, has now completely removed mandatory quarantine and international flight limitations.
As one of the largest in vitro diagnostic exhibitions worldwide, CACLP looks forward to welcoming IVD companies and professionals from around the globe to celebrate the 20th anniversary together.
Limited Impact of COVID-19 in China
¡°China to scrap the quarantine requirement on January 8¡±
¡°Fully reopen borders between Hong Kong, Macao and mainland China¡±
¡°Chinese group tour travel starts again to 20 countries¡±
Over the past few months, China has experienced a rapid and effective recovery from the COVID pandemic and long post-pandemic period.
In the recent Spring Festival travel rush:
- 4.73 billion passenger trips were made and nearly 1.6 billion trips took place via trains, planes and waterways, recovering to 53.5% of pre-pandemic levels in 2019, according to data released by the Ministry of Transport.
- The number of people crossing the border of the Chinese mainland reached up to 676,000 per day, as the National Immigration Administration revealed.
The data on increasing travel confirms a considerable step on the road back to normality, that COVID-19 is no longer an obstacle to transnational exchange in the fields of tourism, economy and technology. Meanwhile, the further brightened economic background and declined demands of COVID-related products bring the IVD industry new opportunities and challenges.
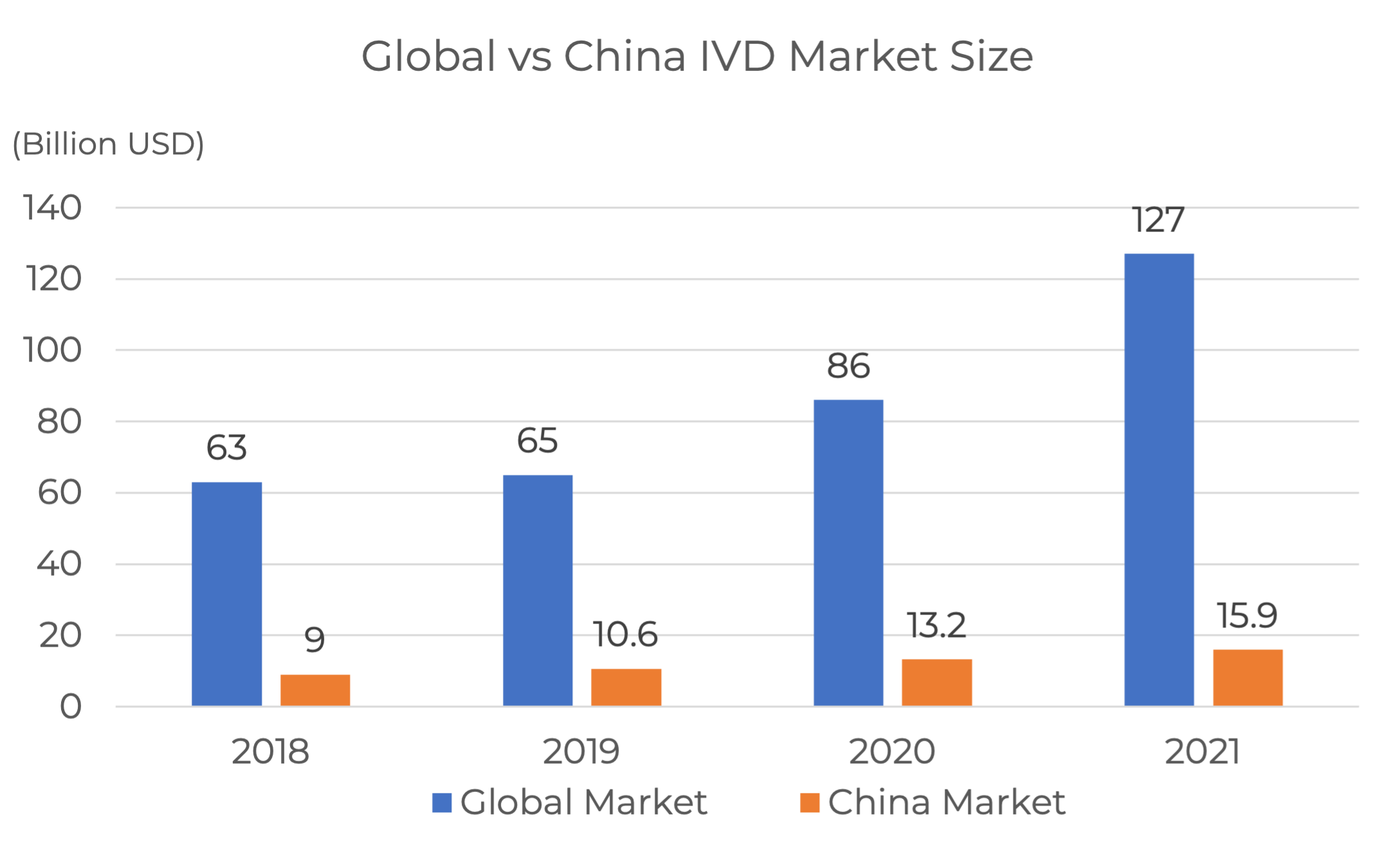
Growth Prospect in China IVD Market
China IVD market had reached USD 15.9 billion by 2021 which accounted for 12.5% of the global, maintaining a high growth rate last year (Annual Report on the Data of Medical Device Industry in China, 2022).
Data released in FY2022 financial results of domestic and international healthcare enterprises shows that the coronavirus pandemic has boosted profits of the diagnostics sector, and most IVD companies still affirmed their forecast for revenue growth in 2023 through actively shifting the focus of business and production to product and service innovations.
China is undoubtedly a booming IVD market, as the government declared in the Healthy China 2030 blueprint that public health is a precondition for all future economic and social development.
¡°With the policy support of Health China and the huge medical market demand continuing to grow, with growing public health expenditure and the aging society, the China IVD industry will continue its positive trend,¡± said Professor Haibo SONG, the chairman of China Association of In-Vitro Diagnostics (CAIVD) and the founder of CACLP.

To consolidate and further develop the graded diagnosis and treatment system and promote the construction of compact urban medical groups in the grid layout, China will continue to promote regional compact urban medical groups by the end of the year to meet people¡¯s whole life cycle health service needs and more third-party independent laboratories will benefit from it.
Additionally, the Chinese government has taken steps to optimize the conditions of overseas investment. On January 11, the General Office of the State Council communicated Measures for Further Encouraging Foreign Investment in the Establishing Research and Development Centers by the Ministry of Commerce and the Ministry of Science and Technology.
The document pointed out that more support should be given to foreign investment in setting up R & D centers in China to carry out scientific and technological R & D and innovation activities. Companies such as Roche, Abbott Laboratories, Beckman Coulter, Siemens Healthineers, Hitachi and PerkinElmer have also accelerated their localization in China by establishing R&D centers and production bases and signing frequent strategic cooperation agreements with domestic companies. We believe more overseas IVD companies can achieve smooth development of business in China with stronger local support and increasing market capacity.
CACLP as a World Leading IVD EXPO
Debut launched in 1991, CACLP is well established as a broad and comprehensive platform that integrates professional exhibitions and academic programs onsite and connects manufacturers, suppliers, end customers, investors, authoritative experts and influencers of head enterprises.
In the past three years of the coronavirus pandemic, CACLP has actively reacted to medical problems and challenges with strong social responsibility. By tightly connecting IVD manufacturers to link supply and demand and holdingwebinars for industry information, medical practices and pandemic advice, CACLP proves itself as an important platform for global IVD players.
With an exhibition space of 130,000 square meters, CACLP 2023 is expected to attract over 30,000 visitors to attend exhibitions, conferences and forums. Thousands of Chinese and international companies have registered to exhibit their cutting-edge products and services.

The upcoming CACLP 2023 welcomes exhibitors and attendees from around the globe to visit us in person to see the dynamic, rapidly developing China IVD market. Our goals is to form a worldwide high-quality IVD community, making the 20th anniversary of CACLP another important moment in the IVD industry.
For more information, please visit: https://en.caclp.com
About GL events
GL events, whose HQ is located in Lyon, was founded in 1978. It is listed on Euronext Paris, Segment B. The core business of GL events covers three business segments, i.e., VENUES (venue management), EXHIBITIONS (exhibition organization) and LIVE (consulting, design and services for events). Through its wide global network of more than 90 offices, with 483 proprietary exhibitions and events, managing 60+ venues and serving 4,200 events, GL events is a critical force for the city promotion of 28 MICE destinations. GL events reported consolidated annual revenue of over €1.1 billion for 2019, which ranked a leading position in the industry.
About CACLP
Debut launched in 1991, CACLP, the China Association of Clinical Laboratory Practice Expo, iss well established as one of the largest exhibitions in the in vitro diagnostic industry worldwide. CISCE, China IVD Supply Chain Expo, successfully launched since 2021, further expands the product sectors from upstream to downstream. With a great number of high-level academic and educational programs held concurrently onsite, and by providing a year-round promotional solution, CACLP proves itself as one of the most important platforms for global IVD players.
Every year, CACLP brings together over 30,000 professionals including entrepreneurs, scholars, users and influencers in the clinical laboratory industry from around the globe to exchange the latest developments in the industry, enhance partnerships and shape the future of the IVD industry.
Contact for press
Sudi FANG
sudi.fang@gl-events.com
Tel: +86 21 5255 8222