



Original from: businesswire
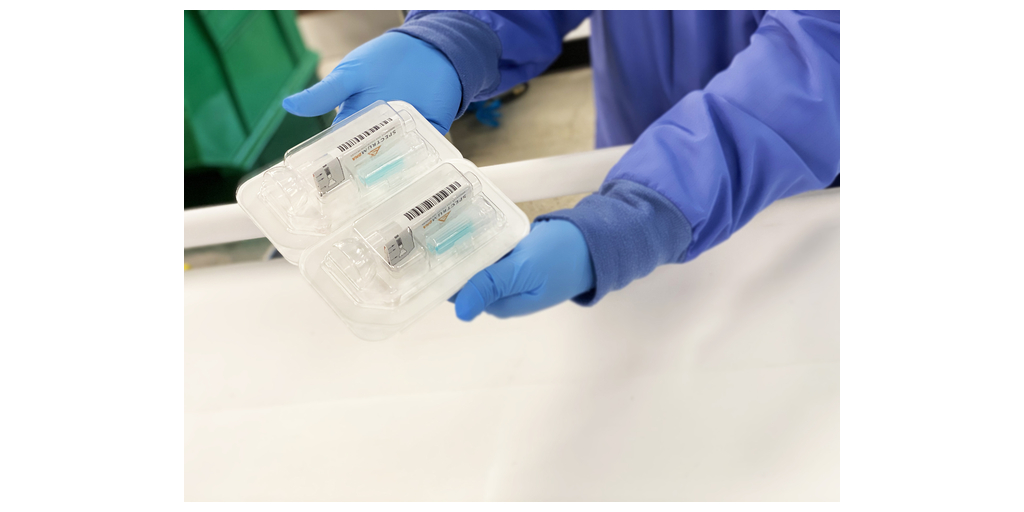
The SDNA device is driving a new era of healthcare that turns to salivary collection and preservation as a means to detect viral infections and resolve the most common points of failure associated with whole saliva. The device is heralded for its ability to maximize detection at the lowest levels and neutralize viruses within 10-seconds of collection to mitigate unnecessary viral exposure. The company¡¯s patented preservation media keeps analytes stable for many weeks at ambient temperatures, ensuring safe, easy, and secure specimen storage and transport. To ensure the highest degree of stringency and quality, automated methods for extraction and validation were performed on industry-leading platforms.
Near the beginning of the pandemic, Spectrum¡¯s SDNA-1000 was the first to gain FDA Emergency Use Authorization (EUA) and introduce a nation, held under viral siege, to the noninvasive, highly accurate, and earliest detection benefits of COVID-19 testing using the self-collection of saliva. With over two years of processing SARS-CoV-2 saliva tests, the device and its patented nucleic acid preservation chemistry have not only demonstrated but proven their unique and superior capabilities deserving of this new device clearance.
¡°This 510(k) clearance and certification as an IVD molecular diagnostic device enables physicians, hospitals, researchers and others to leverage our SDNA Saliva Collection Device in a broad array of FDA approved and LDT diagnostic testing applications,¡± said Stephen Fanning, CEO at Spectrum Solutions. ¡°Those conducting screening and diagnostic tests can confidently and safely expand access and opportunity incorporating saliva as a primary or alternative biomaterial into their existing testing protocols.¡±
¡°We couldn¡¯t be more excited for the opportunity this new 510(k) device clearance delivers in supporting laboratory medicine and healthcare systems in safely and confidently decentralizing specimen collections for precise and accurate testing,¡± said Chief Medical Officer, Rohit Gupta, at Spectrum Solutions.
ABOUT SPECTRUM SOLUTIONS®
Headquartered in Salt Lake City, Utah, Spectrum Solutions is a healthcare solutions partner striving to bridge the gap between the science and real medical solutions. Our laboratory products and services, clinical testing, and onsite compounding pharmacy are driving decentralized testing, accelerating multi-omic scientific applications, modernizing direct-to-consumer wellness, and delivering innovation with the practical power to impact and change outcomes.