



Original from: OraSure Technologies
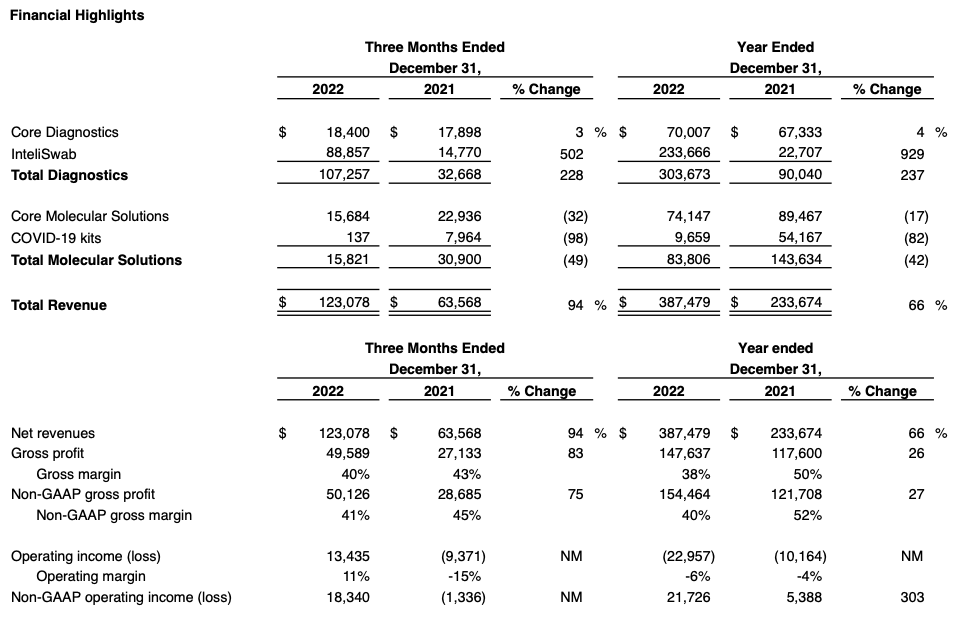
- InteliSwab® revenue of $88.9 million in Q4, up 12% sequentially; wins two new federal government contracts extending the tail on InteliSwab® revenue
- Signs a deal with Quest Diagnostics Genomic Sequencing Services for saliva collection kits
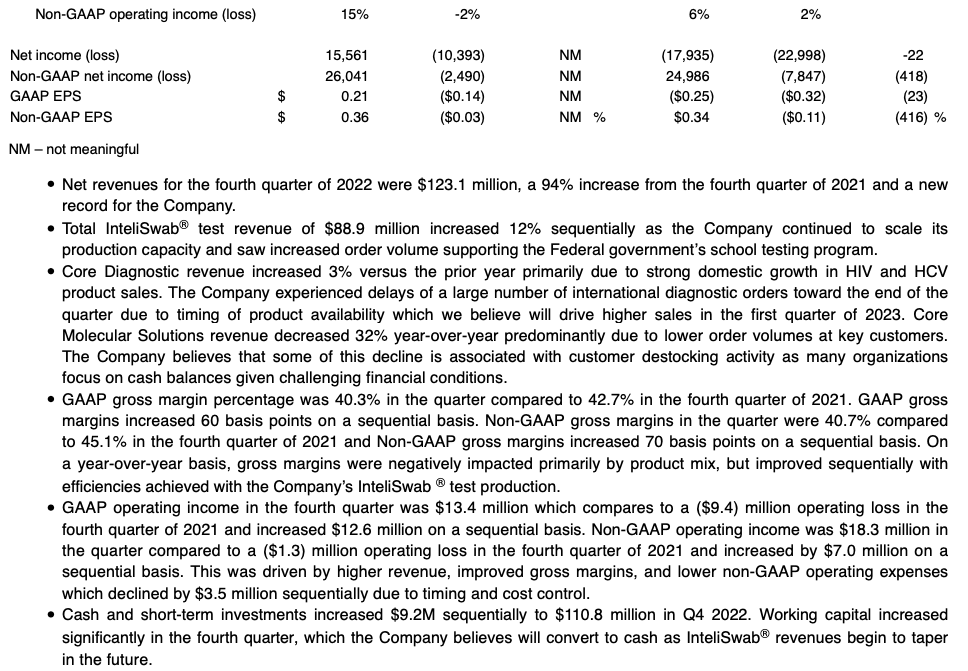
- Q4 GAAP EPS of $0.21; Non-GAAP EPS of $0.36
- Announces restructuring expected to result in operating expense savings of $15 million to be fully implemented by June 2023; targets achieving cash flow breakeven for core business by end of 2024
- Cash balance grows to $111 million, up $9 million from prior quarter
OraSure Technologies, Inc. (NASDAQ: OSUR), a leader in point-of-care and home diagnostic tests, specimen collection devices, and microbiome laboratory and analytical services, announced its financial results for the three months ended December 31, 2022.
¡°This quarter we have delivered clear progress on our transformation journey to Strengthen our foundation. We identified meaningful expense reductions through restructuring our two business units into a single organization, reducing our non-production workforce by 11%, and lowering manufacturing costs. Importantly, this quarter we further increased our cash balance that will help fund investments for our future,¡± said OraSure President and CEO Carrie Eglinton Manner.
She continued, ¡°building from our stronger base, our focus this year is to increasingly Elevate core growth across our product lines by expanding our existing business, driving internal innovation, and targeting key strategic partnerships. We believe our actions will drive the Company to achieving cash flow breakeven for the core business by the end of 2024 while we Accelerate profitable growth.¡±
Business Restructuring
The Company has shared a business restructuring combining its Molecular Solutions and Diagnostics business units into One OraSure. As part of the restructuring, the Company is announcing role eliminations that will affect 11% of its current non-production workforce. The Company believes that, when coupled with additional cost savings, these changes will lead to annualized operating expense reductions of approximately $15 million to be fully implemented by the end of the second quarter of 2023. With additional system changes and manufacturing site consolidation, the Company targets achieving cash flow breakeven on base business (excluding InteliSwab® revenue) by the end of calendar year 2024. The Company¡¯s profitability turnaround enables current cash generation from InteliSwab® to be utilized for innovation and future growth investments.
¡°To support our long-term goals and growth as an organization, we¡¯ve made the difficult decision to eliminate a number of roles,¡± said OraSure President and CEO Carrie Eglinton Manner. ¡°The changes we made will streamline levels of leadership, centralize core enterprise functions, and prioritize value creation activities. We believe our new operating structure will unlock significant operating efficiencies, foster stronger collaboration across the organization, and allow us to increase our innovation pipeline for growth.¡±
Recent Business Highlights
- Launched four new CE-IVD Collipee urine collection products to support growing interest in non-invasive HPV and STI screening diagnostics and liquid biopsy from first-void urine samples. Included among these launches is a product with a proprietary chemistry for cell-free DNA and extra-cellular vesicles for oncology applications.
- Chosen as one of a group of manufacturers to win a Connecticut tender that allows the Company to compete for a total award of six million potential InteliSwab® tests annually.
Financial Guidance
The Company is guiding toward 1Q23 revenue of $125 to $130 million representing 85% to 92% growth relative to the first quarter of last year. Given the continued volatility with the COVID-19 market, OraSure is only providing quarterly financial guidance for fiscal year 2023; however, it is anticipating higher revenue in the first half of the year followed by lower sales in the second half as the Company works down its COVID-19 government contracts.
About OraSure Technologies
OraSure Technologies empowers the global community to improve health and wellness by providing access to accurate, essential information. OraSure, together with its wholly-owned subsidiaries, DNA Genotek, Diversigen, and Novosanis, provides its customers with end-to-end solutions that encompass tools, services and diagnostics. The OraSure family of companies is a leader in the development, manufacture, and distribution of rapid diagnostic tests, sample collection and stabilization devices, and molecular services solutions designed to discover and detect critical medical conditions. OraSure¡¯s portfolio of products is sold globally to clinical laboratories, hospitals, physician¡¯s offices, clinics, public health and community-based organizations, research institutions, government agencies, pharma, commercial entities and direct to consumers.